Organocadmium chemistry
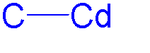
An organocadmium compound is a organometallic compound containing a carbon to cadmium chemical bond. Organocadmium chemistry describes physical properties, synthesis, reactions and use of these compounds.[1] Cadmium shares group 12 with zinc and mercury and their corresponding chemistries have much in common.
Dimethylcadmium is a linear molecule with C-Cd bond lengths of 211.2 pm. All organocadmium compounds are sensitive to air, light and moisture.
Synthesis
The first organocadmium compounds, dimethylcadmium, CH3-Cd-CH3, and diethylcadmium, CH3CH2-Cd-CH2CH3, were prepared in 1917 by Erich Krause. In general, they are prepared by transmetalation or by an exchange reaction between an organometallic reagent and a cadmium salt.[2]
One procedure for the synthesis of diethylcadmium is by the reaction of cadmium bromide with two equivalents of the Grignard reagent ethylmagnesium bromide in diethyl ether. A byproduct is magnesium bromide. Diethylcadmium is a colorless oil with melting point −21 °C.
Diphenylcadmium can be prepared by the reaction of phenyllithium with cadmium bromide. Diphenylcadmium is a solid with a melting point of 174 °C.
Reactions
The synthetic utility of organocadmiums is limited. The alkyl groups in them are less nucleophilic than the organozincs due to the general increase in electronegativity going down group 12. This reduced reactivity is demonstrated in the conversion of acid chlorides to ketones with these reagents.[3] This reaction was discovered by Henry Gilman in 1936 and was used until less toxic cuprates were available. With other organometallic reagents such reaction would continue to the corresponding alcohol.
An example of the synthetic use of an organocadmium is the reaction of diisoamylcadmium with β-carbomethoxypropionyl chloride to methyl 4-keto-7-methyloctanoate without reacting further with the ketone group or the ester group.[4]
Another example is the use of methyl cadmium in one of the steps leading to cholesterol total synthesis:[5]

Dimethylcadmium is also used in the synthesis of colloidal nanocrystals although its toxic and volatile nature has led researchers to look elsewhere for cadmium precursors such as cadmium oxide.[6][7]
This selectivity exists provided that the reaction is carried out salt free.[8] When the cadmium reagent is generated in situ from a cadmium salt, the presence of a halide salt makes the reagent much more reactive, even to ketones. The same salt effect can be observed with organozinc compounds.
See also
- Other chemistries of carbon with other group 12 elements: organozinc compounds, organocadmium compounds and organomercury compounds.
References
- ^ Synthetic Methods of Organometallic and Inorganic Chemistry Vol 5, Copper, Silver, Gold, Zinc, Cadmium, and Mercury W.A. Herrmann Ed. ISBN 3-13-103061-5
- ^ Erich Krause (1917). "Einfache Cadmiumdialkyle. (I. Mitteilung über organische Cadmium-Verbindungen.)". Berichte der deutschen chemischen Gesellschaft. 50 (2): 1813–1822. doi:10.1002/cber.19170500292.
- ^ James Cason (1947). "The Use of Organocadmium Reagents for the Preparation of Ketones". Chemical Reviews. 40 (1): 15–32. doi:10.1021/cr60125a002. PMID 20287882.
- ^ Organic Syntheses, Coll. Vol. 3, p.601 (1955); Vol. 28, p.75 (1948) Article
- ^ The Total Synthesis of Steroids R. B. Woodward, Franz Sondheimer, David Taub, Karl Heusler, W. M. McLamore J. Am. Chem. Soc., 1952, 74 (17), pp 4223–4251 doi:10.1021/ja01137a001
- ^ Formation of High-Quality CdTe, CdSe, and CdS Nanocrystals Using CdO as Precursor Peng and Peng, J. Am. Chem. Soc. 2001, 123, 183-184
- ^ [1]
- ^ The less familiar reactions of organocadmium reagents Paul R. Jones and Peter J. Desio Chem. Rev.; 1978; 78(5) pp 491 - 516 doi:10.1021/cr60315a001
