Organotitanium chemistry

Organotitanium compounds in organometallic chemistry contain carbon-to-titanium chemical bonds. Organotitanium chemistry is the science of organotitanium compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry and are involved in major industrial processes.[1][2]
Brief history
Although the first attempt to create an organotitanium compound dates back to 1861, it took until 1953 for the first synthesis of such a compound. In that year titanium phenyltriisopropoxide was prepared from titanium isopropoxide, phenyllithium, and titanium tetrachloride. Titanocene dichloride was discovered in 1954, and the first methyltitanium compounds were produced in 1959. Ziegler-Natta catalysts utilizing titanium-based catalysts soon followed as a major commercial application for which the 1963 Nobel Prize in Chemistry was awarded.
Properties
The titanium electron configuration ([Ar]3d24s2) resembles that of carbon and like carbon the +4 oxidation state dominates and like carbon compounds, those of titanium have a tetrahedral molecular geometry. Thus, the boiling points of TiCl4 and CCl4 are very similar. Titanium is however a much larger element than carbon, reflected by the Ti-C bond lengths being about 30% longer, e.g. 210 pm in tetrabenzyltitanium vs a typical C-C bond of 155 pm. Simple tetraalkyltitanium compounds however are not typically stable, owing to the large size of titanium and the electron-deficient nature of its tetrahdral complexes. More abundant and more useful than the simple tetraalkyl compounds are organic derivatives with alkoxide and cyclopentadienyl coligands. Titanium is capable of forming complexes with high coordination numbers.
In terms of oxidation states, most organotitanium chemistry, in solution at least, focuses on derivatives of Ti(IV). Ti(II) compounds are rarer, examples being titanocene dicarbonyl and Ti(CH3)2(dmpe)2. [Ti(CO)6]2− is formally a complex of Ti(-II).[3] Although Ti(III) is involved in Ziegler-Natta catalysis, the organic derivatives of Ti(III) are not common, though the dimer [Cp2TiIIICl]2 is well known.[4]
The oxidation states −1, 0, +1 are also known in organotitanium compounds.[5][6][7]
Due to the low electronegativity of titanium, Ti-C bonds are polarized toward carbon. Consequently, alkyl ligands in many titanium compounds are nucleophilic. Titanium is characteristically oxophilic, which presents challenges to handling these compounds, which require air-free techniques. On the other hand, high oxophilicity means that titanium alklyls are effective for abstracting or exchanging organyl ligands for oxo groups, as discussed below.
Chemistry
Organotitanium compounds are important reagents in organic chemistry.[8] Some reagents include the following.
- The Ziegler–Natta catalyst (1954) is obtained from titanium(III) chloride and diethylaluminium chloride and important in ethylene polymerization.
- Methyltitanium trichloride CH3TiCl3 (1959) is a nonbasic nucleophilic reagent. It can be prepared by reacting titanium(IV) chloride with dimethylzinc in dichloromethane at -78 °C. It is used in nucleophilic addition of methyl groups to carbonyl compounds and in SN1 methylation of alkyl halides. Methyltriisopropoxytitanium is a related reagent prepared in situ from titanium isopropoxide, titanium(IV) chloride and methyllithium[9]
- The Kulinkovich reaction is a cyclopropanation method starting from a Grignard reagent and an ester. The first step is transmetallation forming a dialkyltitanium intermediate.
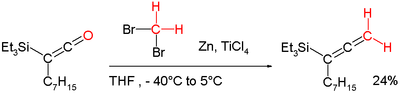
- Lombardo's reagent is a carbenoid methylenation reagent (see Tebbe reagent below).,[10] which is a low temperature version of the Dibromomethane-Zinc-Titanium(IV) Chloride reagent[11] was developed to overcome a shortcoming of the Wittig reagent by methylenating enolisable carbonyl groups without loss of stereochemical integrity (Lombardo Methylenation). It can for example also be applied in a conversion of a ketene into an allene:[8][12]
Titanocene derivatives

A particularly rich area of organotitanium chemistry involves derivatives of titanocene dichloride.[13] Early work on "titanocene" itself eventually revealed that this species was a fulvalene dimer complex.[13][15] The titanocene dimer was recognised in the 1970s[15][16][17] but not structurally characterised until 1992,[14] and the investigations led to many innovations on cyclopentadienyl complexes of titanium.[13] Only in 1998 was a true titanocene derivative identified, the paramagnetic species (C5Me4SiMe3)2Ti.[18]
Tebbe's reagent (1978) is prepared from titanocene dichloride and trimethylaluminium. It is used as a methylenation agent for carbonyl compounds (converstion of R2C=O to R2C=CH2). It is an alternative for Wittig reagents when the carbonyl group is sterically challenged or when it easily forms the enol.[8] Tebbe's reagent itself does not react with carbonyl compounds, but must first be treated with a mild Lewis base, such as pyridine, which generates the active Schrock carbene.
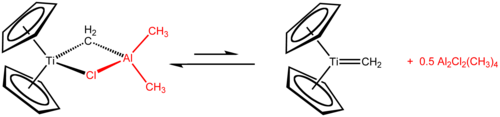
Tebbe's reagent adds simple alkenes to give titanocyclobutanes, which can be regarded as stable olefin metathesis intermediates. These compounds are reagents in itself such as 1,1-bis(cyclopentadienyl)-3,3-dimethyltitanocyclobutane, the adduct of Tebbe's reagent with isobutene catalysed with 4-dimethylaminopyridine.[19]

The Petasis reagent or dimethyl titanocene (1990) is prepared from titanocene dichloride and methyllithium in diethyl ether. Compared to Tebbe's reagent it is easier to prepare and easier to handle. It is also a methylenation reagent.[19]
The Nugent-RajanBabu reagent[20] is a one-electron reductant used in synthetic organic chemistry for the generation of alcohols via anti-Markovnikov ring-opening of epoxides, and is generated as a dimer [(η5-Cp)2Ti(μ-Cl)]2 and used in situ from titanocene dichloride.[4][21][22][23]
See also
References
- ^ "Encyclopedia of Reagents for Organic Synthesis", L.A. Paquette, Ed.: J. Wiley and Sons: Sussex, England, 1996
- ^ "Organotitanium Reagents in Organic Synthesis (Reactivity and Structure Concepts in Organic Chemistry, Vol 24)" Manfred T. Reetz 1986 ISBN 0-387-15784-0
- ^ Elschenbroich, C. "Organometallics" (2006) Wiley-VCH: Weinheim. ISBN 978-3-527-29390-2
- ^ a b Manzer, L. E.; Mintz, E. A.; Marks, T. J. (1982). "Cyclopentadienyl Complexes of Titanium(III) and Vanadium(III)". Inorg. Synth. 21: 84–86. doi:10.1002/9780470132524.ch18.
- ^ "A New Approach to Bis(arene)titanium(0) and -titanium(–I) Complexes; Structure of Bis(arene)titanates(1–)". Angewandte Chemie International Edition in English. 31 (3): 1495–1498. November 1992. doi:10.1002/anie.199214951.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Synthesis of [Ti(η6-1,3,5-C6H3iPr3)2][BAr4] (Ar = C6H5, p-C6H4F, 3,5-C6H3(CF3)2), the First Titanium(I) Derivatives". Organometallics. 16 (14): 3100–3101. 1997. doi:10.1021/om970155o.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Synthesis of anionic sandwich compounds: [Ti(η-C6H5R)2]– and the crystal structure of [K(18-crown-6)(µ-H)Mo(η-C5H5)2]". J. Chem. Soc., Chem. Commun.: 729–731. 1984. doi:10.1039/C39840000729.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ a b c Hartwig, J. F. Organotransition Metal Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010. ISBN 1-891389-53-X
- ^ Organic Syntheses, Coll. Vol. 8, p.495 (1993); Vol. 67, p.180 (1989) Link.
- ^ Organic Syntheses, Coll. Vol. 8, p.386 (1993); Vol. 65, p.81 (1987) Link.
- ^ Takai, K.; Hotta, Y.; Oshima, K.; Nozaki, H. Tetrahedron Lett. 1978, 2417-2420.
- ^ "Synthesis of highly substituted allenylsilanes by alkylidenation of silylketenes" Stephen P Marsden and Pascal C Ducept Beilstein Journal of Organic Chemistry 2005, 1:5 doi:10.1186/1860-5397-1-5
- ^ a b c d Mehrotra, R. C.; Singh, A. (2000). "4.3.6 η5-Cyclopentadienyl d-Block Metal Complexes". Organometallic Chemistry: A Unified Approach (2nd ed.). New Delhi: New Age International Publishers. pp. 243–268. ISBN 9788122412581.
- ^ a b Troyanov, Sergei I.; Antropiusová, Helena; Mach, Karel (1992). "Direct proof of the molecular structure of dimeric titanocene; The X-ray structure of μ(η5:η5-fulvalene)-di-(μ-hydrido)-bis(η5-cyclopentadienyltitanium)·1.5 benzene". J. Organomet. Chem. 427 (1): 49–55. doi:10.1016/0022-328X(92)83204-U.
- ^ a b Wailes, P. C.; Coutts, R. S. P.; Weigold, H. (1974). "Titanocene". Organometallic Chemistry of Titanium, Zirconium, and Hafnium. Organometallic Chemistry. Academic Press. pp. 229–237. ISBN 9780323156479.
- ^ Antropiusová, Helena; Dosedlová, Alena; Hanuš, Vladimir; Karel, Mach (1981). "Preparation of μ-(η5:η5-Fulvalene)-di-μ-hydrido-bis(η5-cyclopentadienyltitanium) by the reduction of Cp2TiCl2 with LiAlH4 in aromatic solvents". Transition Met. Chem. 6 (2): 90–93. doi:10.1007/BF00626113.
- ^ Cuenca, Tomas; Herrmann, Wolfgang A.; Ashworth, Terence V. (1986). "Chemistry of oxophilic transition metals. 2. Novel derivatives of titanocene and zirconocene". Organometallics. 5 (12): 2514–2517. doi:10.1021/om00143a019.
- ^ Chirik, Paul J. (2010). "Group 4 Transition Metal Sandwich Complexes: Still Fresh after Almost 60 Years". Organometallics. 29 (7): 1500–1517. doi:10.1021/om100016p.
- ^ a b Hartley, Richard C.; Li, Jianfeng; Main, Calver A.; McKiernan, Gordon J. (2007). "Titanium carbenoid reagents for converting carbonyl groups into alkenes". Tetrahedron. 63 (23): 4825–4864. doi:10.1016/j.tet.2007.03.015.
- ^ Rosales, Antonio; Rodríguez-Garcia, Ignacio; Muñoz-Bascón, Juan; Roldan-Molina, Esther; Padial, Natalia M.; Morales, Laura P.; García-Ocaña, Marta; Oltra, J. Enrique (2015). "The Nugent Reagent: A Formidable Tool in Contemporary Radical and Organometallic Chemistry". Eur. J. Org. Chem. 2015 (21): 4567–4591. doi:10.1002/ejoc.201500292.
This review article was corrected to refer to the "Nugent–RajanBabu Reagent" rather than the "Nugent Reagent" by:
Rosales, Antonio; Rodríguez-Garcia, Ignacio; Muñoz-Bascón, Juan; Roldan-Molina, Esther; Padial, Natalia M.; Morales, Laura P.; García-Ocaña, Marta; Oltra, J. Enrique (2015). "The Nugent–RajanBabu Reagent: A Formidable Tool in Contemporary Radical and Organometallic Chemistry". Eur. J. Org. Chem. 2015 (21): 4592. doi:10.1002/ejoc.201500761. - ^ Handa, Yuichi; Inanaga, Junji (1987). "A highly stereoselective pinacolization of aromatic and α, β-unsaturated aldehydes.dta mediated by titanium(III)-magnesium(II) complex". Tetrahedron Lett. 28 (46): 5717–5718. doi:10.1016/S0040-4039(00)96822-9.
- ^ Nugent, William A.; RajanBabu, T. V. "Transition-metal-centered radicals in organic synthesis. Titanium(III)-induced cyclization of epoxy olefins". J. Am. Chem. Soc. 110 (25): 8561–8562. doi:10.1021/ja00233a051.
- ^ Jungst, Rudolph; Sekutowski, Dennis; Davis, Jimmy; Luly, Matthew; Stucky, Galen (1977). "Structural and magnetic properties of di-μ-chloro-bis[bis(η5-cyclopentadienyl)titanium(III)] and di-μ-bromo-bis[bis(η5-methylcyclopentadienyl)titanium(III)]". Inorg. Chem. 16 (7): 1645–1655. doi:10.1021/ic50173a015.