Pentanes
Appearance
The pentanes are a group of alkanes with five carbon atoms with the formula C5H12. It has three isomers.
| Common name | normal pentane unbranched pentane n-pentane |
isopentane | neopentane |
| IUPAC name | pentane | 2-methylbutane | 2,2-dimethylpropane |
| Molecular diagram | 
|

|
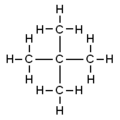
|
| Skeletal diagram | 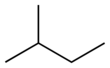
|

| |
| Melting Point (°C)[1] | −129.8 | −159.9 | −16.6 |
| Boiling Point (°C)[1] | 36.0 | 27.7 | 9.5 |
| Density (0°C,kg/m3)[1] |
621 | 616 | 586 |
References
- ^ a b c James Wei (1999), Molecular Symmetry, Rotational Entropy, and Elevated Melting Points. Ind. Eng. Chem. Res., volume 38 issue 12, pp. 5019–5027 doi:10.1021/ie990588m
