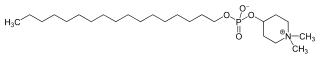
Perifosine
| |

| |
| Names | |
|---|---|
| IUPAC name
1,1-Dimethylpiperidinium-4-yl octadecyl phosphate
| |
| Other names
D 21266; KRX 0401
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.217.789 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C25H52NO4P | |
| Molar mass | 461.668 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Perifosine (also KRX-0401) is a drug candidate being developed for a variety of cancer indications. It is an alkyl-phospholipid[1] structurally related to miltefosine. It acts as an Akt inhibitor and a PI3K inhibitor. It was being developed by Keryx Biopharmaceuticals who have licensed it from Æterna Zentaris Inc.[2]
In 2010 perifosine has reached phase II.[3] In one phase II trial for metastatic colon cancer perifosine doubled time to progression.[2]
It has orphan drug status in the U.S. for the treatment of multiple myeloma and neuroblastoma, and for multiple myeloma in the EU.[4]
In 2011 it was in a phase III trial for colorectal cancer,[5] and another for multiple myeloma.[4][6] On April 2, 2012, it was announced that perifosine failed its phase III clinical trial for treatment of colon cancer.[7] Detailed results were released in June 2012.[8] On March 11, 2013 Aeterna Zentaris announced the discontinuing of Phase 3 clinical trial of perifosine for the treatment of relapsed and refractory multiple myeloma http://www.aezsinc.com/en/page.php?p=60&q=550.
References
- ^ Kondapaka; et al. (Nov 2003). "Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation". Mol Cancer Ther.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b Smartoncology newsletter, Feb 2010
- ^ June 7, 2010: Presentation at the American Society of Clinical Oncology annual meeting of Phase I data on single agent perifosine in the treatment of recurrent pediatric solid tumors, including patients with advanced brain tumors and neuroblastoma. also Phase II trial of the novel oral Akt inhibitor perifosine in relapsed and/or refractory Waldenstrom macroglobulinemia (WM).
- ^ a b "Yakult Pays Aeterna Zentaris $8.3M for Japanese Rights to Pivotal-Stage Cancer Drug". 9 March 2011.
- ^ http://www.clinicaltrial.gov/ct2/show/NCT01097018
- ^ http://www.clinicaltrial.gov/ct2/show/NCT01002248
- ^ "Aeterna Zentaris Regains North American Rights to Akt Inhibitor from Keryx". May 2012.
- ^ "Aeterna Zentaris: Phase 3 Data for Perifosine in Colorectal Cancer Presented at ASCO Meeting". 4 June 2012.
