Phosphine ligand

Phosphine ligands are phosphines, compound of the formula PRR'R" (R, R', R" = H, alkyl, aryl, etc) that are used as ligands in metal complexes, often related to organometallic chemistry and homogeneous catalysis. These compounds are also used in other areas of chemistry.[1]
Common ligands
The most common phosphine ligands are of the type PR3. They are three-fold symmetric with equivalent substituents. Some routine phosphine ligands are triphenylphosphine and trimethylphosphine. The triarylphosphines are usually white shelf-stable solids, whereas the trialkylphosphines are colorless liquids that tend to air-oxidize to the corresponding phosphine oxides (R3PO). Such ligands can be classified according to their donor strength and steric bulk. These properties can be quantified by the Tolman electronic parameter and ligand cone angle, respectively. Generally alkyl phosphines are stronger bases and σ-donors.
Chelating phosphines
Bidentate phosphines
Common bidentate chelating phosphine ligands include dppe and dmpe, R2PCH2CH2PR2 (R = Ph, Me, respectively).
Tridentate phosphines
Tridentate triphosphines come in two classes, linear and tripodal. These ligands are both (confusingly) called triphos. The phenyl-substituted versions have the formula CH3C(CH2PPh2)3 and PhP(CH2CH2PPh2)2.
Tetradentate phosphines
Examples of tetradentate tripodal phosphines include tris[2-(diphenylphosphino)ethyl]phosphine (pp3).[2]
Chiral phosphine ligands
Two basic types of chiral phosphine ligands exist. These are of interest for asymmetric catalysis, e.g., asymmetric hydrogenation. Chiral diphosphines have been particularly popularized. P-Chiral phosphines such as DIPAMP have three different phosphorus substituents. BINAP is a well known example of a C2-symmetric diphosphine which forms chiral complexes due to atropisomerism.
| sPhos | 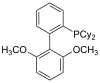
|
SPANphos | 
|
| SEGphos | 
|
Triphos | |
| Xantphos | 
|
Kwon phosphine | 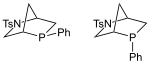
|
| Chiraphos | 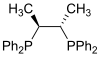
|
duPhos | 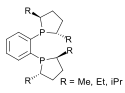
|
| A selection of phos ligands | |||
References
- ^ Downing, J.H.; Smith, M.B. "Phosphorus Ligands". Comprehensive Coordination Chemistry II. 2003: 253–296. doi:10.1016/B0-08-043748-6/01049-5.
- ^ Ghilardi, C. A.; Midollini, S.; Sacconi, L. (May 2002). "Reactions of the tripod ligand tris(2-diphenylphosphinoethyl)phosphine with cobalt(II) and nickel(II) salts and sodium borohydride. Structural characterization of a five-coordinate cobalt(I) hydride complex". Inorganic Chemistry. 14 (8): 1790–1795. doi:10.1021/ic50150a010.
