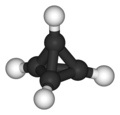
Platonic hydrocarbon
Appearance
Platonic hydrocarbons are the molecular representation of platonic solid geometries with vertices replaced by carbon atoms and with edges replaced by chemical bonds. Not all platonic solids have a molecular counterpart:
- Tetravalent carbon excludes an icosahedron (5 edges meeting at each vertex) as a feasible objective;
- Angle strain prohibits an octahedron[1] . Since 4 edges meet at each corner, there would be no hydrogen atoms, and this hypothetical octahedron molecule would be an allotrope C6 of elemental carbon, not a hydrocarbon.
The following platonic hydrocarbons, on the other hand, have been synthesised:
- Tetrahedrane (C4H4) but only with proper substituents;
- Cubane (C8H8);
- Dodecahedrane (C20H20).
Note that with increasing number of carbon atoms in the frame, the geometry will eventually approximate a sphere. This is ultimately accomplished in fullerene although not a Platonic hydrocarbon itself (Buckminsterfullerene, C60, has the shape of a truncated icosahedron, an Archimedean solid).
-
Tetrahedrane
-
Cubane
-
Dodecahedrane
References
- ^ Nevertheless, note the existence of [1.1.1.1]paddlane C(CH2)4C, CAS number 102843-69-6 and the theorical existence of pyramidane which is an half octahedron with one carbon on a vertex like those of octahedron.
- Henning Hopf, Classics in Hydrocarbon Chemistry, Wiley VCH, 2000.
Wikimedia Commons has media related to platonic hydrocarbons.