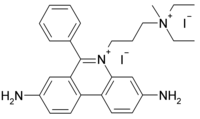
Propidium iodide
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.042.786 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C27H34I2N4 | |
| Molar mass | 668.3946 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Propidium iodide (or PI) is an intercalating agent and a fluorescent molecule with a molecular mass of 668.4 Da that can be used to stain cells. When PI is bound to nucleic acids, the fluorescence excitation maximum is 535 nm and the emission maximum is 617 nm. Excitation energy can be supplied with a xenon or mercury-arc lamp or with the 488 line of an argon-ion laser.[1] Propidium iodide is used as a DNA stain for both flow cytometry, to evaluate cell viability or DNA content in cell cycle analysis,[2] and microscopy to visualise the nucleus and other DNA-containing organelles. It can be used to differentiate necrotic, apoptotic and normal cells.[3]
Propidium Iodide is the most commonly used dye to quantitatively assess DNA content.[4][citation needed]
Propidium iodide (PI) binds to DNA by intercalating between the bases, with little or no sequence preference, and with a stoichiometry of one dye per 4–5 base pairs of DNA. PI also binds to RNA, necessitating treatment with nucleases to distinguish between RNA and DNA staining.[5] Once the dye is bound to nucleic acids, its fluorescence is enhanced 20- to 30-fold, the fluorescence excitation maximum is shifted ~30–40 nm to the red, and the fluorescence emission maximum is shifted ~15 nm to the blue. Although its molar absorptivity (extinction coefficient) is relatively low, PI exhibits a sufficiently large Stokes shift to allow simultaneous detection of nuclear DNA and fluorescein-labeled antibodies, provided the proper optical filters are used. PI is suitable for fluorescence microscopy, confocal laser scanning microscopy, flow cytometry, and fluorometry.
PI is membrane impermeant and generally excluded from viable cells. PI is commonly used for identifying dead cells in a population and as a counterstain in multicolor fluorescent techniques.[6] The counterstaining protocols below are compatible with a wide range of cytological labeling techniques—direct or indirect antibody-based detection methods, mRNA in situ hybridization, or staining with fluorescent reagents specific for cellular structures. These protocols can be modified for tissue staining.
A typical use of propidium iodide in plant biology is to stain the cell wall. Especially useful for Arabidopsis thaliana seedling root tissue observed by confocal microscopy, it increases visibility of the outlines of cells in the root tip. This red fluorescent background is useful to determine the sub-localization of a gene of interest expressed as a Green Fluorescent Protein fusion.
Also, propidium iodide is used as a stain in animal cells. For example, in Apodemus sylvaticus, more commonly known as the wood mouse, it can be used to indicate the location of the nuclear region of sperm cell by emitting its characteristic red fluorescence.[7]
See also
References
- ^ http://probes.invitrogen.com/media/pis/mp01304.pdf
- ^ "Propidium Iodide Solution - BioLegend". Retrieved 10 January 2015.
- ^ Lecoeur H (2002). "Nuclear apoptosis detection by flow cytometry: influence of endogenous endonucleases". Exp. Cell Res. 277 (1): 1–14. doi:10.1006/excr.2002.5537. PMID 12061813.
- ^ Cancer Research UK. 2004. Cell Cycle Analysis - Propidium Iodide. http://science.cancerresearchuk.org/sci/facs/facs_major_apps/cell_cycle_analysis/propidium_iodide/?version=1
- ^ Suzuki T, Fujikura K, Higashiyama T, Takata K (1 January 1997). "DNA staining for fluorescence and laser confocal microscopy". J. Histochem. Cytochem. 45 (1): 49–53. doi:10.1177/002215549704500107. PMID 9010468.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Moore A, Donahue CJ, Bauer KD, Mather JP (1998). "Simultaneous measurement of cell cycle and apoptotic cell death". Methods Cell Biol. 57: 265–78. doi:10.1016/S0091-679X(08)61584-8. PMID 9648110.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Moore, Harry et al., Exceptional sperm cooperation in Wood Mouse.Nature 418, 174-177 (2002).
