Propyl gallate
| |
| |
| Names | |
|---|---|
| IUPAC name
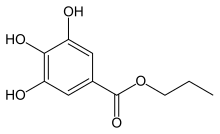
Propyl 3,4,5-trihydroxybenzoate
| |
| Other names
Gallic acid, propyl ester
n-Propyl gallate E310 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.090 |
| EC Number |
|
| E number | E310 (antioxidants, ...) |
| MeSH | Propyl+Gallate |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H12O5 | |
| Molar mass | 212.20 g/mol |
| Appearance | White crystalline powder |
| Melting point | 150 °C (302 °F; 423 K) |
| Boiling point | Decomposes |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Propyl gallate, or propyl 3,4,5-trihydroxybenzoate is an ester formed by the condensation of gallic acid and propanol. Since 1948, this antioxidant has been added to foods containing oils and fats to prevent oxidation.[1] As a food additive, it is used under the E number E310.
Description
Propyl gallate is an antioxidant. It protects against oxidation by hydrogen peroxide and oxygen free radicals.
Uses
Propyl gallate is used to protect oils and fats in products from oxidation; it is used in foods, cosmetics, hair products, adhesives, and lubricants.
It is used as a triplet state quencher and an antioxidant in fluorescence microscopy.[2]
Biological effects
A 1993 study in fat rodents found little or no effect on carcinogenesis by propyl gallate.[3]
A 2009 study found that propyl gallate acts as an estrogen antagonist.[4]
References
- ^ "Final Report on the Amended Safety Assessment of Propyl Gallate". International Journal of Toxicology. 26 (suppl. 3): 89–118. 2007. doi:10.1080/10915810701663176. ISSN 1091-5818. PMID 18080874.
- ^ Jerker Widengren; Andriy Chmyrov; Christian Eggeling; Per-Åke Löfdahl; Claus A. M. Seidel (2007). "Strategies to Improve Photostabilities in Ultrasensitive Fluorescence Spectroscopy". The Journal of Physical Chemistry A. 111 (3): 429–440. doi:10.1021/jp0646325. PMID 17228891.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help) - ^ Hirose, Masao, et al. "Modification of carcinogenesis by α-tocopherol, t-butylhydro-quinone, propyl gallate and butylated hydroxytoluene in a rat multi-organ carcinogenesis model." Carcinogenesis 14.11 (1993): 2359-2364.
- ^ Alessio Amadasi; Andrea Mozzarelli; Clara Meda; Adriana Maggi; Pietro Cozzini (2009). "Identification of Xenoestrogens in Food Additives by an Integrated in Silico and in Vitro Approach". Chem. Res. Toxicol. 22 (1): 52–63. doi:10.1021/tx800048m. PMC 2758355. PMID 19063592.
