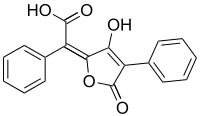
Pulvinic acid
| |
| Names | |
|---|---|
| IUPAC name
(2E)-(5-Hydroxy-3-oxo-4-phenyl-2(3H)-furanylidene)(phenyl)acetic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C18H12O5 | |
| Molar mass | 308.289 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pulvinic acid is a natural chemical pigment found in some lichens.[1] Dimers of pulvinic acid have been found in the fungi Scleroderma citrinum and Chalciporus piperatus.[2]
See also
References
- ^ Bourdreux, Yann; Bodio, Ewen; Willis, Catherine; Billaud, Célia; Le Gall, Thierry; Mioskowski, Charles (2008). "Synthesis of vulpinic and pulvinic acids from tetronic acid". Tetrahedron. 64 (37): 8930–8937. doi:10.1016/j.tet.2008.06.058.
- ^ Winner M, Giménez A, Schmidt H, Sontag B, Steffan B, Steglich W (2004). "Unusual pulvinic acid dimers from the common fungi Scleroderma citrinum (common earthball) and Chalciporus piperatus (peppery bolete)". Angewandte Chemie. 43 (14): 1883–6. doi:10.1002/anie.200352529. PMID 15054803.
