Quaternary ammonium cation
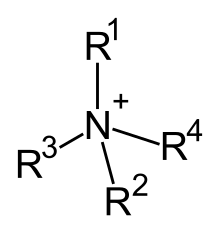
Quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure NR4+ with R being alkyl groups. Unlike the ammonium ion NH4+ itself and primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium cations are synthesized by complete alkylation of ammonia or other amines. For possible synthesis route, see amines.
Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations with an anion. They are used as disinfectants, surfactants, fabric softeners, and as antistatic agents (e.g. in shampoo). In liquid fabric softeners, the chloride salts are often used. In dryer anticling strips, the sulfate salts are often used. This is also a common ingredient in many spermicidal jellies.
In organic chemistry, quaternary ammonium salts are used as phase transfer catalysts for reactions involving immiscible solvent systems, such as the synthesis of dichlorocarbene with chloroform and sodium hydroxide.
The synthesis of this cation from ammonia is referred to as quaternization.
Through exhaustive methylation, or the Hofmann Elimination process, a quaternary ammonium iodide salt is formed. The alpha-carbon (relative to the nitrogen) is deprotonated once by a hydroxide anion from H2O and the electrons form an alkene. Subsequently, the electrons from the carbon-nitrogen bond are pushed onto the nitrogen. This sets up a tertiary amine as the leaving group.[1]
Antimicrobial use
Quats are most effective against gram-positive bacteria. Also good against fungi, amoeba, and enveloped viruses.[2] Quats act by disrupting the cell membrane and proteins. Quats kill just about everything except endospores, Mycobacterium tuberculosis, lipid-containing viruses, and Pseudomonas spp. (some Pseudomonas spp. can even grow in solutions of quats, subsisting on them).
In contrast to phenolics, quats are not very effective in the presence of organic compounds. Yet quats are very effective in combination with phenols. Quats are deactivated by soaps, other anionic detergents, and cotton fibers.[3] Also, they are not recommended to be used in hard water. Effective levels are at 200 ppm.[4] They are effective at temperatures up to 212 ºF.[5]
References
- ^ www.organic-chemistry.org
- ^ Specific Antimicrobials, outline of lecture by Stephen T. Abedon, Ohio State U., URL accessed Dec 2008.
- ^ Specific Antimicrobials, outline of lecture by Stephen T. Abedon, Ohio State U., URL accessed Dec 2008.
- ^ The Use of Disinfectants In the Swine Industry, Mark G. Ladd, North Carolina State Univ., URL accessed Dec 2008.
- ^ Matching the Right Disinfectant to the Job, Michelle Gardner, URL accessed Dec 2008.
See also
- Choline
- Carnitine
- Benzalkonium chloride, benzethonium chloride, methylbenzethonium chloride, cetalkonium chloride, cetylpyridinium chloride, cetrimonium, cetrimide, dofanium chloride, tetraethylammonium bromide, and domiphen bromide; antimicrobial ingredients found in various over-the-counter products
- Denatonium; the most bitter compound known
- Cetyl trimethylammonium bromide (CTAB), stearalkonium chloride; cationic surfactants commonly used in toiletries
- Cocamidopropyl betaine (CAPB), a very common Zwitterionic surfactant used ubiquitously in toiletries
- Tetra-n-butylammonium bromide and Aliquat 336; common phase transfer catalysts
- Polyquaternium; designations for quaternary ammonium-containing polymers used for personal care products
- Paraquat; an herbicide
- Quaternary ammonium muscle relaxants
