Radiation: Difference between revisions
m Reverted 1 edit by 92.43.64.85 identified as vandalism to last revision by 96.247.95.86. (TW) |
|||
| Line 6: | Line 6: | ||
There are three principal types of ionizing radiation: [[Alpha decay|alpha]], [[Beta decay|beta]] and [[Gamma ray|gamma]] radiation. They are all emitted from the nucleus of an unstable atom. Less commonly encountered are spontaneous [[nuclear fission]], [[positron emission]], and [[neutron emission]]. [[Electron capture]] results in the spontaneous emission of an X-ray. Certain isotopes of [[radium]] have a decay mode where they emit an entire [[Carbon-12|<sup>12</sup>C<sub>6</sub>]] nucleus. |
There are three principal types of ionizing radiation: [[Alpha decay|alpha]], [[Beta decay|beta]] and [[Gamma ray|gamma]] radiation. They are all emitted from the nucleus of an unstable atom. Less commonly encountered are spontaneous [[nuclear fission]], [[positron emission]], and [[neutron emission]]. [[Electron capture]] results in the spontaneous emission of an X-ray. Certain isotopes of [[radium]] have a decay mode where they emit an entire [[Carbon-12|<sup>12</sup>C<sub>6</sub>]] nucleus. |
||
dsfdsfdiduuduedudufydy8fdusyfusfddshfdhsfhf |
|||
==Discovery== |
|||
[[Wilhelm Röntgen]] is credited with the discovery of [[X-Rays]]. When experimenting with various isotopes of tritium, he noticed a drastic change in photonic emissions when measuring electrical charges in a vacuum. When he took pictures of the tritium, he found that the state of one solid piece would deteriorate quickly. [[Henri Becquerel]] found that [[uranium]] salts caused fogging of an unexposed photographic plate, and [[Marie Curie]] discovered that only certain elements gave off these rays of energy. She named this behaviour [[radioactivity]]. |
|||
In December of 1899, Marie Curie and [[Pierre Curie]] discovered radium in pitchblende. This new element was two million times more radioactive than uranium, as described by Marie. |
|||
== See also == |
== See also == |
||
Revision as of 09:07, 10 October 2008
Radiation, as in physics, is energy in the form of waves or moving subatomic particles emitted by an atom or other body as it changes from a higher energy state to a lower energy state. Radiation can be classified as ionizing or non-ionizing radiation, depending on its effect on atomic matter. The most common use of the word "radiation" refers to ionizing radiation. Ionizing radiation has enough energy to ionize atoms or molecules while non-ionizing radiation does not. Radioactive material is a physical material that emits ionizing radiation.
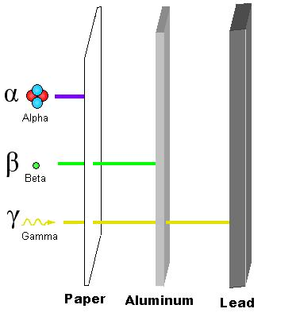
Types of Radiation
There are three principal types of ionizing radiation: alpha, beta and gamma radiation. They are all emitted from the nucleus of an unstable atom. Less commonly encountered are spontaneous nuclear fission, positron emission, and neutron emission. Electron capture results in the spontaneous emission of an X-ray. Certain isotopes of radium have a decay mode where they emit an entire 12C6 nucleus.
dsfdsfdiduuduedudufydy8fdusyfusfddshfdhsfhf
See also
- Ionizing radiation
- Non-ionizing radiation
- Background radiation, which actually refers to the background ionizing radiation
- Black hole
- Cosmic microwave background radiation, 3K blackbody radiation that fills the Universe
- Electromagnetic spectrum
- Radiant energy, radiation by a source into the surrounding environment.
- Radiation damage - adverse effects on materials and devices
- Radiation hormesis - dosage threshold damage theory
- Radiation poisoning - adverse effects on life forms
- Radiation hardening - making devices resistant to failure in high radiation environments
- Radioactive contamination
- Radioactive decay
- Hawking radiation
- Cherenkov radiation
- Cyclotron radiation
- Solar radiation
- Synchrotron radiation
- Radiation reaction
- National Council on Radiation Protection and Measurements
