Reduction of nitro compounds
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.
Aromatic nitro compounds[edit]
Reduction to anilines[edit]
The reduction of nitroaromatics is conducted on an industrial scale.[1] Many methods exist, such as:
- Catalytic hydrogenation using: Raney nickel[2] or palladium-on-carbon,[3][4][5] platinum(IV) oxide, or Urushibara nickel.[6]
- Iron in acidic media.[7][8][9]
- Sodium hydrosulfite[10]
- Sodium sulfide (or hydrogen sulfide and base). Illustrated by the selective reduction of dinitrophenol to the nitroaminophenol.[11]
- Tin(II) chloride[12]
- Titanium(III) chloride
- Samarium[13]
- Hydroiodic acid[14]
Metal hydrides are typically not used to reduce aryl nitro compounds to anilines because they tend to produce azo compounds. (See below)
Reduction to hydroxylamines[edit]
Several methods have been described for the production of aryl hydroxylamines from aryl nitro compounds:
- Raney nickel and hydrazine at 0-10 °C[15]
- Electrolytic reduction[16]
- Zinc metal in aqueous ammonium chloride[17]
- Catalytic Rhodium on carbon with excess hydrazine monohydrate at room temperature [18]
Reduction to hydrazine compounds[edit]
Treatment of nitroarenes with excess zinc metal results in the formation of N,N'-diarylhydrazine.[19]
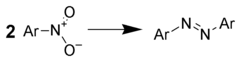
Reduction to azo compounds[edit]
Treatment of aromatic nitro compounds with metal hydrides gives good yields of azo compounds. For example, one could use:
- Lithium aluminium hydride[20]
- Zinc metal with sodium hydroxide.[19] (Excess zinc will reduce the azo group to a hydrazino compound.)
Aliphatic nitro compounds[edit]
Reduction to hydrocarbons[edit]
Hydrodenitration (replacement of a nitro group with hydrogen) is difficult to achieve but can be effected by catalytic hydrogenation over platinum on silica gel at high temperatures.[21] The reaction can also be effected through radical reaction with tributyltin hydride and a radical initiator, AIBN as an example.[22]
Reduction to amines[edit]
Aliphatic nitro compounds can be reduced to aliphatic amines by several reagents:
- Catalytic hydrogenation using platinum(IV) oxide (PtO2)[23] or Raney nickel[24]
- Iron metal in refluxing acetic acid[25]
- Samarium diiodide[26]
- Raney nickel, platinum on carbon, or zinc dust and formic acid or ammonium formate[6]
α,β-Unsaturated nitro compounds can be reduced to saturated amines by:
- Catalytic hydrogenation over palladium-on-carbon
- Iron metal
- Lithium aluminium hydride[27] (Note: Hydroxylamines and oximes are typical impurities.)
- Lithium borohydride or sodium borohydride and trimethylsilyl chloride[28]
- Red-Al[29]
Reduction to hydroxylamines[edit]
Aliphatic nitro compounds can be reduced to aliphatic hydroxylamines using diborane.[30]
The reaction can also be carried out with zinc dust and ammonium chloride:[31][32][33]
- R-NO2 + 4 NH4Cl + 2 Zn → R-NH-OH + 2 ZnCl2 + 4 NH3 + H2O
Reduction to oximes[edit]
Nitro compounds are typically reduced to oximes using metal salts, such as tin(II) chloride[34] or chromium(II) chloride.[35] Additionally, catalytic hydrogenation using a controlled amount of hydrogen can generate oximes.[36]
References[edit]
- ^ Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411. ISBN 978-3527306732.
- ^ Allen, C. F. H.; VanAllan, J. (1955). "2-Amino-p-cymene". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 3, p. 63. - ^ Bavin, P. M. G. (1973). "2-Aminofluorene". Organic Syntheses; Collected Volumes, vol. 5, p. 30.
- ^ Smith, Michael B.; March, Jerry (2007). March's Advanced Organic Chemistry (6th ed.). John Wiley & Sons. p. 1816. ISBN 978-0-471-72091-1.
- ^ Ram, Siya; Ehrenkaufer, Richard E. (1984). "A general procedure for mild and rapid reduction of aliphatic and aromatic nitro compounds using ammonium formate as a catalytic hydrogen transfer agent". Tetrahedron Letters. 25 (32): 3415–3418. doi:10.1016/S0040-4039(01)91034-2. hdl:2027.42/25034.
- ^ a b Adams, J. P. (2002). "Nitro and related groups". Journal of the Chemical Society, Perkin Transactions 1 (23): 2586–2597. doi:10.1039/b009711j.
- ^ Fox, B. A.; Threlfall, T. L. (1964). "2,3-Diaminopyridine". Organic Syntheses. 44: 34. doi:10.15227/orgsyn.044.0034
{{cite journal}}: CS1 maint: multiple names: authors list (link). - ^ Mahood, S. A.; Schaffner\doi=10.15227/orgsyn.011.0032, P. V. L. (1931). "2,4-Diaminotoluene". Organic Syntheses. 11: 32. doi:10.15227/orgsyn.011.0032.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ "O-Aminobenzaldehyde, Redox-Neutral Aminal Formation and Synthesis of Deoxyvasicinone". Organic Syntheses. 89: 274. 2012. doi:10.15227/orgsyn.089.0274.
- ^ Redemann, C. T.; Redemann, C. E. (1955). "5-Amino-2,3-dihydro-1,4-phthalazinedione". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 3, p. 69. - ^ Hartman, W. W.; Silloway, H. L. (1945). "2-Amino-4-nitrophenol". Organic Syntheses. 25: 5. doi:10.15227/orgsyn.025.0005.
- ^ Faul, Margaret M.; Thiel, Oliver R. (2005). "Tin(II) Chloride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt112.pub2. ISBN 9780470842898.
- ^ Basu, M. K. (2000). "Ultrasound-promoted highly efficient reduction of aromatic nitro compounds to the aromatic amines by samarium/ammonium chloride". Tetrahedron Lett. 41 (30): 5603–5606. doi:10.1016/S0040-4039(00)00917-5.
- ^ Kumar, J. S. Dileep; Ho, ManKit M.; Toyokuni, Tatsushi (2001). "Simple and chemoselective reduction of aromatic nitro compounds to aromatic amines: reduction with hydriodic acid revisited". Tetrahedron Letters. 42 (33): 5601–5603. doi:10.1016/s0040-4039(01)01083-8.
- ^ Ayyangar, N. R.; Brahme, K. C.; Kalkote, U. R.; Srinivasan, K. V. (1984). "Facile Transfer-Reduction of Nitroarenes to N Arylhydroxylamines with Hydrazine in the Presence of Raney Nickel". Synthesis. 1984 (11): 938. doi:10.1055/s-1984-31027.
- ^ Harman, R. E. (1963). "Chloro-p-benzoquinone". Organic Syntheses; Collected Volumes, vol. 4, p. 148.
- ^ Kamm, O. (1941). "β-Phenylhydroxylamine". Organic Syntheses; Collected Volumes, vol. 1, p. 445.
- ^ Ichikawa, S.; Zhu, S.; Buchwald, S. (2018). "A Modified System for the Synthesis of Enantioenriched N-Arylamines through Copper-Catalyzed Hydroamination". Angewandte Chemie International Edition. 57 (28): 8714–8718. doi:10.1002/anie.201803026. hdl:1721.1/125726. PMC 6033674. PMID 29847002.
- ^ a b Bigelow, H. E.; Robinson, D. B. (1955). "Azobenzene". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 3, p. 103. - ^ R. F. Nystrom & W. G. Brown (1948). "Reduction of Organic Compounds by Lithium Aluminum Hydride. III. Halides, Quinones, Miscellaneous Nitrogen Compounds". J. Am. Chem. Soc. 70 (11): 3738–3740. doi:10.1021/ja01191a057. PMID 18102934.
- ^ M. J. Guttieri & W. F. Maier (1984). "Selective cleavage of carbon-nitrogen bonds with platinum". J. Org. Chem. 49 (16): 2875–2880. doi:10.1021/jo00190a006.
- ^ T. V. (Babu) RajanBabu, Philip C. Bulman Page, Benjamin R. Buckley, "Tri-n-butylstannane" Encyclopedia of Reagents for Organic Synthesis 2004, John Wiley & Sons. doi:10.1002/047084289X.rt181.pub2
- ^ A. T. Nielsen (1962). "The Isomeric Dinitrocyclohexanes. II. Stereochemistry". J. Org. Chem. 27 (6): 1998–2001. doi:10.1021/jo01053a019.
- ^ Dauben, Jr., H. J.; Ringold, H. J.; Wade, R. H.; Pearson, D. L.; Anderson, Jr., A. G. (1963). "Cycloheptanone". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 4, p. 221. - ^ Senkus, M. (1948). "Iron Reduction of Some Aliphatic Nitro Compounds". Ind. Eng. Chem. 40: 506. doi:10.1021/ie50459a035.
- ^ A. S. Kende & J. S. Mendoza (1991). "Controlled reduction of nitroalkanes to alkyl hydroxylamines or amines by samarium diiodide". Tetrahedron Letters. 32 (14): 1699–1702. doi:10.1016/S0040-4039(00)74307-3.
- ^ A. Burger, M. L. Stein and J. B. Clements (1957). "Some Pyridylnitroalkenes, Nitroalkanols, and Alkylamines". J. Org. Chem. 22 (2): 143–144. doi:10.1021/jo01353a010.
- ^ Giannis, A.; Sandhoff, K. (1989). "LiBH4(NaBH4)/Me3SiCl, an Unusually Strong and Versatile Reducing Agent". Angewandte Chemie International Edition in English. 28 (2): 218–220. doi:10.1002/anie.198902181.
- ^ Butterick, John R.; Unrau, A. M. (1974). "Reduction of β-nitrostyrene with sodium bis-(2-methoxyethoxy)-aluminium dihydride. A convenient route to substituted phenylisopropylamines". J. Chem. Soc., Chem. Commun. (8): 307–308. doi:10.1039/c39740000307.
- ^ H. Feuer, R. S. Bartlett, B. F. Vincent and R. S. Anderson (1965). "Diborane Reduction of Nitro Salts. A New Synthesis of N-Monosubstituted Hydroxylamines". J. Org. Chem. 30 (9): 2880–2882. doi:10.1021/jo01020a002.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Smith, P.W.G.; Tatchell, A.R. (1965), "Aliphatic Nitro Compounds and Amines", Fundamental Aliphatic Chemistry, Elsevier, pp. 245–266, doi:10.1016/b978-0-08-010746-2.50016-8, ISBN 978-0-08-010746-2, retrieved 2021-01-27
- ^ Kelly, Sean M.; Lipshutz, Bruce H. (2014-01-03). "Chemoselective Reductions of Nitroaromatics in Water at Room Temperature". Organic Letters. 16 (1): 98–101. doi:10.1021/ol403079x. ISSN 1523-7060. PMC 4013784. PMID 24341483.
- ^ Ung, Stéphane; Falguières, Annie; Guy, Alain; Ferroud, Clotilde (August 2005). "Ultrasonically activated reduction of substituted nitrobenzenes to corresponding N-arylhydroxylamines". Tetrahedron Letters. 46 (35): 5913–5917. doi:10.1016/j.tetlet.2005.06.126.
- ^ Braun, V. J.; Sobecki, W. (1911). "Über primäre Dinitro-, Nitronitrit- und Dialdoxim-Verbindungen der Fettreihe". Chemische Berichte. 44 (3): 2526–2534. doi:10.1002/cber.19110440377.
- ^ J. R. Hanson & E. Premuzic (1967). "Applications of chromous chloride--II : The reduction of some steroidal nitro-compounds". Tetrahedron. 23 (10): 4105–4110. doi:10.1016/S0040-4020(01)97921-9.
- ^ C. Grundmann (1950). "Über die partielle Reduktion von Nitro-cyclohexan". Angewandte Chemie. 62 (23–24): 558–560. Bibcode:1950AngCh..62..558G. doi:10.1002/ange.19500622304.