Saponin
Saponins are a class of chemical compounds, one of very many secondary metabolites found in natural sources, with saponins found in particular abundance in various plant species. Specifically, they are amphipathic glycosides grouped phenomenologically by the soap-like foaming they produce when shaken in aqueous solutions, and structurally by their being composed of one or more hydrophilic glycoside moieties combined with a lipophilic triterpene derivative (see Hostettmann & Marston 1995[1], and Cornell 2008[2]). A ready and therapeutically relevant example is the cardio-active agent digoxin, from common foxglove.
Structural Variety and Biosynthesis
The aglycone (glycoside-free portion) of the saponins are termed sapogenins. The number of saccharide chains attached to the sapogenin/aglycone core can vary—giving rise to another dimension of nomenclature (monodesmosidic, bidesmosidic, etc., Hostettmann, op. cit.)—as can the length of each chain. A somewhat dated compilation has the range of saccharide chain lengths being 1-11, with the numbers 2-5 being the most frequent, and with both linear and branched chain saccharides being represented (ibid.). Dietary monosaccharides such as D-glucose and D-galactose are among the most common components of the attached chains (ibid.).
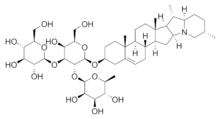
The lipophilic aglycone can be any one of a wide variety of polycyclic organic structures originating from the serial addition of ten-carbon (C10) terpene units to compose a C30 triterpene skeleton (e.g., beta-amyrin, Dixon 2008[3], MetaCyc 2008a[4], and references therein), often with subsequent alteration to produce a C27 steroidal skeleton (Hostettmann, op. cit.). The subset of saponins that are steroidal have been termed saraponins; see Cornell, op. cit. Aglycone derivatives can also incorporate nitrogen, so that some saponins also present chemical and pharmacologic characteristics of alkaloid natural products. The figure at right above presents the structure of the alkaloid phytotoxin solanine, a monodesmosidic, branched-saccharide steroidal saponin. (The lipophilic steroidal structure is the series of connected six- and five-membered rings at the right of the structure, while the three oxygen-rich sugar rings are at left and below. Note the nitrogen atom inserted into the steroid skeleton at right.)
Sources, Especially from Plants, and Localizations Therein
Saponins have historically been understood to be plant-derived, but they have also been isolated from marine organisms (Hostettmann, op. cit., Riguera 1997[5], and therein). Saponins are indeed found in many plants (Birk et al. 1980[6], Hostettmann, op. cit.), and derive their name from the soapwort plant (Genus Saponaria, Family Caryophyllaceae), the root of which was used historically as a soap (Cornell, op. cit.). Saponins are also found in the botanical family Sapindaceae, with its defining genus Sapindus (soapberry or soapnut), and in the families Aceraceae (maples) and Hippocastanaceae (horse chestnuts; ref. needed). Within these families, this class of chemical compounds are found in various parts of the plant: leaves, stems, roots, bulbs, blossom, and fruit (ref. needed). Commercial formulations of plant-derived saponins—e.g., from the soap bark (or soapbark) tree, Quillaja saponaria, and from other sources—are available via controlled manufacturing processes, which make them of use as chemical and biomedical reagents (Sigma-Aldrich 2008[7], and references therein).
Role in Plant Ecology, and Impact on Animal Foraging
In plants, saponins may serve as anti-feedants (Cornell, op. cit., MetaCyc 2008a, op. cit.), and to protect the plant against microbes and fungi (ref. needed). Some plant saponins (e.g. from oat and spinach) may enhance nutrient absorption and aid in animal digestion. However, saponins are often bitter to taste, and so can reduce plant palatability (e.g., in livestock feeds), or even imbue them with life-threatening animal toxicity (ibid.). Data make clear that some saponins are toxic to cold-blooded organisms and insects at particular concentrations (MetaCyc 2008a, op. cit.). There is a need for further research to define the roles of these natural products in their host organisms—which have been described as "poorly understood" to date (ibid.).
Established Research Bioactivities, and Cautions Regarding Therapeutic Claims
Bioactivities. One research use of the saponin class of natural products involves their complexation with cholesterol to form pores in cell membrane bilayers, e.g., in red cell (erythrocyte) membranes, where complexation leads to red cell lysis (hemolysis) on intravenous injection (Francis et al. 2002[8]). In addition, the amphipathic nature of the class gives them activity as surfactants that can be used to enhance penetration of macromolecules such as proteins through cell membranes (Sigma-Aldrich, op. cit., and references therein). Saponins have also has been used as adjuvants in vaccines (ibid.).
Medical uses. There is tremendous, commercially driven promotion of saponins as dietary supplements and nutriceuticals. There is evidence of the presence of saponins in traditional medicine preparations (Asl & Hosseinzadeh 2008[9], Xu et al. 1996[10]), where oral administrations might be expected to lead to hydrolysis of glycoside from terpenoid (and obviation of any toxicity associated with the intact molecule). But as is often the case with wide-ranging therapeutic claims for natural products:
- the claims for organismal/human benefit are often based on very preliminary biochemical or cell biological studies (that is, only the most preliminary of data; scrutinize referenced bioactivity citations in MetaCyc 2008b[11]); and
- mention is generally omitted of the possibilities of individual chemical sensitivity, or to the general toxicity of specific agents (for the Quillaja molina saponin extract, see JTBaker 2008[12]), and high toxicity of selected cases (e.g., solanine above).
While such statements require constant review (and despite the myriad of web claims to the contrary), it appears that there are very limited US, EU, etc. agency-approved roles for saponins in human therapy. In their use as adjuvants in the production of vaccines, toxicity associated with sterol complexation remains a major issue for attention (Skene & Sutton 2006[13]). Even in the case of digoxin, therapeutic benefit from the cardiotoxin is a result of careful administration of an appropriate dose. Very great care needs to be exercised in evaluating or acting on specific claims of therapeutic benefit from ingesting saponin-type and other natural products.
See also
References
- ^ Hostettmann, K. and Marston, A. Saponins: Chemistry and Pharmacology of Natural Products. Cambridge University Press, Cambridge, UK, 3ff, (1995)
- ^ Poisonous plant to livestock: Saponin. Accessed 9 September 2008.
- ^ Project Summary: Functional Genomics of Triterpene Saponin Biosynthesis in Medicago Truncatula. Accessed 10 September 2008.
- ^ MetaCyc Pathway: saponin biosynthesis I. Accessed 10 September 2008.
- ^ Riguera, R. (1997). Isolating bioactive compounds from marine organisms. Journal of Marine Biotechnology. 5: 187-193.
- ^ Birk, Y. and Peri, I., Liener, I.E., ed. Toxic Constituents of Plant Foodstuffs, New York , 161, (1980) [To be checked.]
- ^ Saponin from quillaja bark. Accessed 9 September 2008.
- ^ Francis, G., Kerem, Z., Makkar, H.P., and Becker, K. (2002). The biological action of saponins in animal systems: a review. Br J Nutr 88, 587-605.
- ^ Asl, M.N. and Hosseinzadeh, H. (2008) Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytotherapy research 22(6):709-24.
- ^ Xu, R., Zhao, W., Xu, J., Shao, B., Qin, G. (1996) Studies on bioactive saponins from Chinese medicinal plants. Advances in Experimental Medicine and Biology 404:371-82.
- ^ MetaCyc Pathway: saponin biosynthesis IV. Accessed 10 September 2008.
- ^ Saponin. Accessed 10 September 2008.
- ^ Skene, C.D., Sutton, P. 2006. Saponin-adjuvanted particulate vaccines for clinical use. Methods. 40(1):53-9.
External links
- Medical Dictionary on Saponin
- Saponins in Wine, by ScienceDaily, accessed Sep 9,2003
- Molecular Expressions Phytochemical Gallery - Saponin
- Saponins: Suprising benefits of desert plants
- How to survive the world's worst diet
- Quillia Extracts JECFA Food Additives Series 48
