Schottky defect
A Schottky defect is a type of point defect in a crystal lattice named after Walter H. Schottky.
The defect forms when oppositely charged ions leave their lattice sites, creating vacancies. These vacancies are formed in stoichiometric units, to maintain an overall neutral charge in the ionic solid. The vacancies are then free to move about as their own entities. Normally these defects will lead to a decrease in the density of the crystal. The following are the chemical equations in Kröger-Vink Notation for the formation of Schottky defects in TiO2 and BaTiO3.
Ø
Ø
This can be illustrated schematically with a two-dimensional diagram of a sodium chloride crystal lattice:

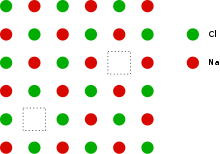
Definition
If in an ionic crystal of type A+B- an equal number of cations and anions are missing from their lattice sites so that electrical neutrality as well as stoichiometry is maintained this is called a Schottky Defect.
It is a vacancy defect (due to missing ions) and also a stoichiometric defect, as the ratio of the number of cations and anions remains the same. It occurs only when there is small difference in size between cations and anions.
Examples
This type of defect is shown in compounds with :
- highly ionic compounds
- high co-ordination number
- small difference in sizes of cations and anions
Examples : NaCl, KCl, CsCl, KBr, AgCl. etc.
Experimental observations show that at room temperature in an NaCl crystal there is one Schottky defect per 1016 ions.
Effect on Density
The total number of ions in a crystal with this defect is less than the theoretical value of ions, thus, the density of the solid crystal is less than normal.
See also
References
Kittel, Charles, Introduction to Solid State Physics - 8th ed. Wiley, 2005. ISBN 0-471-41526-X.


