Schottky defect
A Schottky defect is a type of point defect in a crystal lattice named after Walter H. Schottky. In non-ionic crystals it means a lattice vacancy defect.
In ionic crystals, the defect forms when oppositely charged ions leave their lattice sites, creating vacancies. These vacancies are formed in stoichiometric units, to maintain an overall neutral charge in the ionic solid. The vacancies are then free to move about as their own entities. Normally these defects will lead to a decrease in the density of the crystal. The followings are the chemical equations in Kröger–Vink notation for the formation of Schottky defects in TiO2 and BaTiO3.
- ∅ ⇌ v′′′′
Ti + 2 v••
O
- ∅ ⇌ v′′′′
Ba + v′′′′
Ti + 3 v••
O
This can be illustrated schematically with a two-dimensional diagram of a sodium chloride crystal lattice:

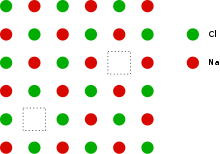
Definition
If in an ionic crystal of type A+B− an equal number of cations and anions are missing from their lattice sites (so that electrical neutrality as well as stoichiometry is maintained), then this defect is called a Schottky defect.
It is a vacancy defect (due to missing ions) and also a stoichiometric defect, as the ratio of the number of cations and anions remains the same.
It occurs only when there is a small difference in size between cations and anions. This is produced as the result of the thermal incorporation of unoccupied lattice sites from the exterior of the crystal. The lattice undergoes thermal vibration and thermal expansion when the temperature is raised above 0 K. When it happens the pair of vacancies are incorporated in the crystal. So electrical neutrality is maintained inside the crystals.
Examples
This type of defect is shown in highly ionic compounds, highly coordinated compounds, and where there is only a small difference in sizes of cations and anions of which the compound lattice is composed.
Effect on density
Since the total number of ions in a crystal with this defect is less than the theoretical number of ions for a crystal of its volume, the density of the solid crystal is less than the theoretical density of the material.
See also
References
- Kittel, Charles (2005). Introduction to Solid State Physics (8th ed.). Wiley. ISBN 0-471-41526-X.[page needed]
