Solanine
This article needs additional citations for verification. (February 2011) |
| |

| |
| Names | |
|---|---|
| IUPAC name
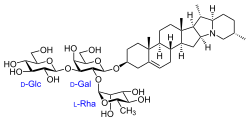
(2S,3R,4S,5S,6R)-2-(((2R,3S,4S,5R,6R)-3-hydroxy-2-(hydroxymethyl)-5-(((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6-(((4S,6aR,6bS,8aS,8bR,9S,9aR,14aS,15aS,15bS)-6a,8a,9-trimethyl-3,4,5,6,6a,6b,7,8,8a,8b,9,9a,10,11,12,13,14a,15,15a,15b-icosahydro-1H-naphtho[2',1':4,5]indeno[1,2-b]indolizin-4-yl)oxy)tetrahydro-2H-pyran-4-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.039.875 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C45H73NO15 | |
| Molar mass | 868.06 |
| Appearance | white crystalline solid |
| Melting point | 271 to 273 °C (520 to 523 °F; 544 to 546 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Solanine is a glycoalkaloid poison found in species of the nightshade family (Solanaceae), such as the potato (Solanum tuberosum) and the tomato (Solanum lycopersicum). It can occur naturally in any part of the plant, including the leaves, fruit, and tubers. Solanine has pesticidal properties, and it is one of the plant's natural defenses. Solanine was first isolated in 1820 from the berries of the European black nightshade (Solanum nigrum), after which it was named.[1]
Solanine poisoning
Symptoms
Solanine poisoning is primarily displayed by gastrointestinal and neurological disorders. Symptoms include nausea, diarrhea, vomiting, stomach cramps, burning of the throat, cardiac dysrhythmia, nightmare, headache and dizziness. In more severe cases, hallucinations, loss of sensation, paralysis, fever, jaundice, dilated pupils, hypothermia and death have been reported.
Ingestion of solanine in moderate amounts can cause death. One study suggests that doses of 2 to 5 mg/kg of body weight can cause toxic symptoms, and doses of 3 to 6 mg/kg of body weight can be fatal.[2]
Symptoms usually occur 8 to 12 hours after ingestion, but may occur as rapidly as 30 minutes after eating high-solanine foods.
Mechanism of action
Solanum glycoalkaloids can inhibit cholinesterase, disrupt cell membranes, and cause birth defects.[3] One study suggests that the toxic mechanism of solanine is caused by the chemical's interaction with mitochondrial membranes. Experiments show that solanine exposure opens the potassium channels of mitochondria, decreasing their membrane potential. This, in turn, leads to Ca2+ being transported from the mitochondria into the cytoplasm, and this increased concentration of Ca2+ in the cytoplasm triggers cell damage and apoptosis.[4]
Correlation with birth defects
Some studies show a correlation between the consumption of potatoes suffering from late blight (which increases solanine and other glycoalkaloid levels) and the incidence of congenital spina bifida in humans.[citation needed] However, other studies have shown no correlation between potato consumption and the incidence of birth defects.[5]
Solanine in potatoes

Solanine occurs naturally in many species of the genus Solanum, including the potato (Solanum tuberosum), tomato (Solanum lycopersicum), eggplant (Solanum melongena), and bittersweet nightshade (Solanum dulcamara).
Potatoes naturally produce solanine and chaconine, a related glycoalkaloid, as a defense mechanism against insects, disease, and herbivores. Potato leaves, stems, and shoots are naturally high in glycoalkaloids.
When potato tubers are exposed to light, they turn green and increase glycoalkaloid production. This is a natural defense to help prevent the uncovered tuber from being eaten. The green colour is from chlorophyll, and is itself harmless. However, it is an indication that increased level of solanine and chaconine may be present. In potato tubers, 30–80% of the solanine develops in and close to the skin, and some potato varieties have high levels of solanine.
Some potato diseases, such as late blight, can dramatically increase the levels of glycoalkaloids present in potatoes. Tubers damaged in harvesting and/or transport also produce increased levels of glycoalkaloids; this is believed to be a natural reaction of the plant in response to disease and damage.
In the 1970s, solanine poisoning affected 78 schoolboys in Britain. Due to immediate and effective treatments, no one died.[6]
Showing green under the skin strongly suggests solanine build-up in potatoes, although each process can occur without the other. A bitter taste in a potato is another, potentially more reliable indicator of toxicity. Because of the bitter taste and appearance of such potatoes, solanine poisoning is rare outside conditions of food shortage. The symptoms are mainly vomiting and diarrhea, and the condition may be misdiagnosed as gastroenteritis. Most potato poisoning victims recover fully, although fatalities are known, especially when victims are undernourished or do not receive suitable treatment.[6] Fatalities are also known from solanine poisoning from other plants in the nightshade family, such as the berries of Solanum dulcamara (woody nightshade).[7]
The United States National Institutes of Health's information on solanine says to never eat potatoes that are green below the skin.[8]
Deep frying potatoes at 170°C (338°F) is known to effectively lower glycoalkaloid levels, because they move into the frying fat, as does boiling, because solanine is water-soluble, while microwave cooking is only somewhat effective, and freeze-drying or dehydration has little effect.[9][10]
Solanine in tomatoes
This section is missing information about tomatoes — do they contain solanine?. (July 2015) |
Some, such as the California Poison Control System, have claimed that tomatoes and tomato leaves contain solanine. However, Dr. Mendel Friedman of the federal Department of Agriculture contradicts this claim, stating that tomatine, a relatively benign alkaloid, is the tomato alkaloid while solanine is found in potatoes. Food science writer Harold McGee has found scant evidence for tomato toxicity in the medical and veterinary literature.[11]
Other uses of solanine
Solanine has fungicidal and pesticidal properties, and solanine hydrochloride (a salt of solanine) has been used as a commercial pesticide, but never on a large scale.[citation needed]
Solanine has sedative and anticonvulsant properties, and has been used as a treatment for asthma, as well as for cough and cold medicines[citation needed]. However, its effectiveness for either use is questionable.[citation needed]
See also
References
- ^ Desfosses, M. (1820): Extrait d'une lettre à M. Robiquet. In: J. de Pharmacie. Bd. 6, S. 374–376.
- ^ Executive Summary of Chaconine & Solanine[dead link]
- ^ Friedman, Mendel; McDonald, Gary M. (1999). "Postharvest Changes in Glycoalkaloid Content of Potatoes". In Jackson, Lauren S.; Knize, Mark G.; Morgan, Jeffrey N. (eds.). Impact of Processing on Food Safety. Advances in Experimental Medicine and Biology. Vol. 459. pp. 121–43. doi:10.1007/978-1-4615-4853-9_9. ISBN 978-1-4615-4853-9. PMID 10335373.
- ^ Gao, Shi-Yong; Wang, Qiu-Juan; Ji, Yu-Bin (2006). "Effect of solanine on the membrane potential of mitochondria in HepG2 cells and [Ca2+]i in the cells". World Journal of Gastroenterology. 12 (21): 3359–67. doi:10.3748/wjg.v12.i21.3359 (inactive 17 August 2015). PMC 4087866. PMID 16733852.
{{cite journal}}: CS1 maint: DOI inactive as of August 2015 (link) CS1 maint: unflagged free DOI (link) - ^ "Solanine and Chaconine". Retrieved 31 May 2009.
- ^ a b "Solanine poisoning". BMJ. 2 (6203): 1458–9. 1979. doi:10.1136/bmj.2.6203.1458-a. PMC 1597169. PMID 526812.
- ^ Alexander, R. F.; Forbes, G. B.; Hawkins, E. S. (1948). "A Fatal Case of Solanine Poisoning". BMJ. 2 (4575): 518. doi:10.1136/bmj.2.4575.518. PMC 2091497. PMID 18881287.
- ^ MedlinePlus Encyclopedia: Potato plant poisoning - green tubers and sprouts
- ^ "Review of Toxicological Literature prepared for Errol Zeiger, PhD, National Institute of Environmental Health Sciences, Submitted by Raymond Tice". Testing Status of Agents at NTP (National Toxicology Program). February 1998. Archived from the original on 17 May 2013.
{{cite web}}:|archive-date=/|archive-url=timestamp mismatch; 18 January 2013 suggested (help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Solanine poisoning from potatoes". FDA Poisonous Plant Database. 1960.
- ^ McGee, Harold (29 July 2009). "Accused, Yes, but Probably Not a Killer". The New York Times. Retrieved 23 May 2010.
External links
- a-Chaconine and a-Solanine, Review of Toxicological Literature
- MedlinePlus Encyclopedia: 002875 — "Green tubers and sprouts"
