Subtelomere
This article needs additional citations for verification. (April 2016) |
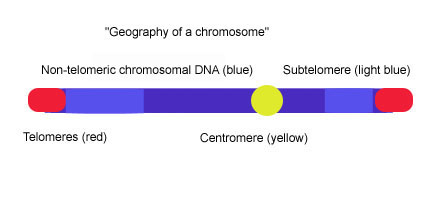
Subtelomeres are segments of DNA between telomeric caps and chromatin.
Structure
Telomeres are specialized protein–DNA constructs present at the ends of eukaryotic chromosomes, which prevent them from degradation and end-to-end chromosomal fusion. Introductory biology courses often describe telomeres as a type of chromosomal aglet. Most vertebrate telomeric DNA consists of long (TTAGGG)n repeats of variable length, often around 3-20kb. Subtelomeres are segments of DNA between telomeric caps and chromatin. Each chromosome has two subtelomeres immediately adjacent to the long (TTAGGG)n repeats. Subtelomeres are considered to be the most distal (farthest from the centromere) region of unique DNA on a chromosome and they are unusually dynamic and variable mosaics of multichromosomal blocks of sequence. The subtelomeres of such diverse species as Humans, Plasmodium falciparum, Drosophila melanogaster or Saccharomyces cerevisiae, are structurally similar in that they are composed of various repeated elements, but the extent of the subtelomeres and the sequence of the elements vary greatly among organisms.[1] In yeast (S. cerevisiae), subtelomeres are composed of two domains : the proximal and distal (telomeric) domains. The two domains differ in sequence content and extent of homology to other chromosome ends and they are often separated by a stretch of degenerate telomere repeats (TTAGGG) and an element called 'core X', which is found at all chromosome ends and contains an autonomously replicating sequence (ARS) and an ABF1 binding site.[2][3] The proximal domain is composed of variable interchromosomal duplications (<1-30 kb), this region can contain genes such Pho, Mel, Mal and open reading frames (ORFs).[4] The distal domain is composed of 0-4 tandem copies of the highly conserved Y' element which contains other ORFs, the number and chromosomal distribution of Y′ elements varies among yeast strains.[5] Between the core X and the Y' element or the core X and TTAGGG sequence there is often a set of 4 'subtelomeric repeats elements' (STR) : STR-A, STR-B, STR-C and STR-D which consists of multiple copies of the vertebrate telomeric motif TTAGGG.[6] This two-domain structure is remarkably similar to the subtelomere structure in human chromosomes 20p, 4q and 18p in which proximal and distal subtelomeric domains are separated by a stretch of degenerate TTAGGG repeats, but, the picture that emerges from studies of the subtelomeres of other human chromosomes indicates that the two-domain model does not apply universally.[1]
Properties
This structure with repeated sequences is responsible for frequent duplication events (which create new genes) and recombination events, at the origin of combination diversity. These peculiar properties are mechanisms that generate diversity at an individual scale and therefore contribute to adaptation of organisms to their environments. For example, in Plasmodium falciparum during interphase of erythrocytic stage, the chromosomic extremities are gathered at the cell nucleus periphery, where they undergo frequent deletion and telomere position effect (TPE). This event in addition to expansion and deletion of subtelomeric repeats, give rise to chromosome size polymorphisms and so, subtelomeres undergo epigenetic and genetic controls. Thanks to the properties of subtelomeres, Plasmodium falciparum evades host immunity by varying the antigenic and adhesive character of infected erythrocytes (see Subtelomeric transcripts).[7][8]
Variations of subtelomere
Variation of subtelomeric regions are mostly variation on STRs, due to recombination of large-scale stretches delimited by (TTAGGG)n-like repeated sequences, which play an important role in recombination and transcription. Haplotype (DNA sequence variants) and length differences are therefore observed between individuals.
Subtelomeric transcripts
Subtelomeric transcripts are pseudogenes (transcribed genes producing RNA sequences not translated into protein) and gene families. In humans, they code for olfactory receptors, immunoglobulin heavy chains, and zinc-finger proteins. In other species, several parasites such as Plasmodium and Trypanosoma brucei have developed sophisticated evasion mechanisms to adapt to the hostile environment posed by the host, such as exposing variable surface antigens to escape the immune system. Genes coding for surface antigens in these organisms are located at subtelomeric regions, and it has been speculated that this preferred location facilitates gene switching and expression, and the generation of new variants.[9][10] For example, the genes belonging to the var family in Plasmodium falciparum (agent of malaria) code for the PfEMP1 (Plasmodium falciparum erythrocyte membrane protein 1), a major virulence factor of erythrocytic stages, var genes are mostly localized in subtelomeric regions. Antigenic variation is orchestrated by epigenetic factors including monoallelic var transcription at separate spatial domains at the nuclear periphery (nuclear pore), differential histone marks on otherwise identical var genes, and var silencing mediated by telomeric heterochromatin. Other factors such as non-coding RNA produced in subtelomeric regions adjacent or within var genes may contribute as well to antigenic variation.[11][12] In Trypanosoma brucei (agent of sleeping sickness), variable surface glycoprotein (VSG) antigenic variation is a relevant mechanism used by the parasite to evade the host immune system. VSG expression is exclusively subtelomeric and occurs either by in situ activation of a silent VSG gene or by DNA rearrangement that inserts an internal silent copy of a VSG gene into an active telomeric expression site. To contrast with Plasmodium falciparum, in Trypanosoma brucei, antigenic variation is orchestrated by epigenetic and genetic factors.[13][14] In Pneumocystis jirovecii major surface glycoprotein (MSG) gene family cause antigenic variation. MSG genes are like boxes at chromosome ends and only the MSG gene at the unique locus UCS (upstream conserved sequence) is transcribed. Different MSG genes can occupy the expression site (UCS), suggesting that recombination can take a gene from a pool of silent donors and install it at the expression site, possibly via crossovers, activating transcription of a new MSG gene, and changing the surface antigen of Pneumocystis jirovecii. Switching at the expression site is probably facilitated by the subtelomeric locations of expressed and silent MSG genes. A second subtelomeric gene family, MSR, is not strictly regulated at the transcriptional level, but may contribute to phenotypic diversity. Antigenic variation in P. jirovecii is dominated by genetic regulation.[15][16]
Pathologic implication
Loss of telomeric DNA through repeated cycles of cell division is associated with senescence or somatic cell aging. In contrast, germ line and cancer cells possess an enzyme, telomerase, which prevents telomere degradation and maintains telomere integrity, causing these types of cells to be very long-lived.
In humans, the role of subtelomere disorders is demonstrated in facioscapulohumeral muscular dystrophy (FSHD), Alzheimer's disease, and peculiar syndromic diseases (malformation and mental retardation). For example, FSHD is associated with a deletion in the subtelomeric region of chromosome 4q. A series of 10 to >100 kb repeats is located in the normal 4q subtelomere, but FSHD patients have only 1–10 repeat units. This deletion is thought to cause disease owing to a position effect that influences the transcription of nearby genes, rather than through the loss of the repeat array itself.[1]
Analysis
Subtelomere analysis, especially sequencing and profiling of patient subtelomeres, is difficult because of the repeated sequences, length of stretches, and lack of databases on the topic.
References
- ^ a b c Mefford HC, Trask BJ (February 2002). "[The complex structure and dynamic evolution of human subtelomeres]". Nature Reviews Genetics. 3: 91–102. doi:10.1038/nrg727. PMID 11836503.
- ^ Louis EJ, Naumova ES, Lee A, Naumov G, Haber JE (1994). "[The chromosome end in yeast: its mosaic nature and influence on recombinational dynamics]". Genetics. 136: 789–802. PMC 1205885. PMID 8005434.
- ^ Walmsley RW, Chan CS, Tye BK, Petes TD (July 1984). "[Unusual DNA sequences associated with the ends of yeast chromosomes]". Nature. 310: 157–160. doi:10.1038/310157a0.
- ^ Coissac E, Maillier E, Robineau S, Netter P (December 1996). "[Sequence of a 39,411 bp DNA fragment covering the left end of chromosome VII of Saccharomyces cerevisiae]". Yeast. 12 (15): 1555–1562. doi:10.1002/(SICI)1097-0061(199612)12:15<1555::AID-YEA43>3.0.CO;2-Q.
- ^ Louis EJ, Haber JE (1992). "[The structure and evolution of subtelomeric Y′ repeats in Saccharomyces cerevisiae]". Genetics. 131: 559–574. PMC 1205030. PMID 1628806.
- ^ Louis EJ (December 1995). "[The chromosome ends of Saccharomyces cerevisiae]". Yeast. 11 (16): 1553–1573. doi:10.1002/yea.320111604.
- ^ Rubio JP, Thompson JK, Cowman AF (1996). "[The var genes of Plasmodium falciparum are located in the subtelomeric region of most chromosomes]". The EMBO Journal. 15 (15): 4069–4077. PMC 452127. PMID 8670911.
- ^ Su XR, Heatwoie VM, Wertheimer SP, Guinet F, Hertfeldt JA, Peterson DS, Ravetch JA, Weilems TE (July 1994). "[The Large Diverse Gene Family var Encodes Proteins Involved in Cytoadherence and Antigenic Variation of Plasmodium falciparum-Infected Erythrocytes]". Cell. 82: 89–100. doi:10.1016/0092-8674(95)90055-1.
- ^ Cano MIN (September 2001). "[Telomere biology of Trypanosomatids: more questions than answers]". Trends in Parasitology. 17 (9, 1): 425–429. doi:10.1016/S1471-4922(01)02014-1.
- ^ Barry JD, Ginger ML, Burton P, McCulloch R (January 2003). "[Why are parasite contingency genes often associated with telomeres?]". International Journal for Parasitology. 33 (1): 29–45. doi:10.1016/S0020-7519(02)00247-3.
- ^ Scherf A, Lopez-Rubio JJ, Riviere L (October 2008). "[Antigenic Variation in Plasmodium falciparum]". Annual Review of Microbiology. 62: 445–470. doi:10.1146/annurev.micro.61.080706.093134.
- ^ Guizetti J, Scherf A (February 2013). "[Silence, activate, poise and switch! Mechanisms of antigenic variation in Plasmodium falciparum]". Cellular Microbiology. 15 (5): 718–726. doi:10.1111/cmi.12115. PMC 3654561.
- ^ Cross GAM (February 2005). "[Antigenic variation in trypansosomes: Secrets surface slowly]". BioEssays. 18 (4): 283–291. doi:10.1002/bies.950180406. PMID 8967896.
- ^ Rudenko G (October 2000). "[The polymorphic telomeres of the African trypanosome Trypanosoma brucei]". Biochemical Society Transactions. 28 (5): 536–540. doi:10.1042/bst0280536. PMC 3375589. PMID 11044370.
- ^ Stringer JR (2014). Edward JL, Becker MM (eds.). [Pneumocystis carinii Subtelomeres]. pp. 101–115. doi:10.1007/978-3-642-41566-1_5.
- ^ Stringer JR, Keely SP (February 2001). "[Genetics of Surface Antigen Expression in Pneumocystis carinii]". Infection and Immunity. 69 (2): 627–639. doi:10.1128/iai.69.2.627-639.2001. PMC 97933. PMID 11159949.
External links
- The flow of genetic information—PDF file. See Table 5.5

