Sulfamide
Appearance
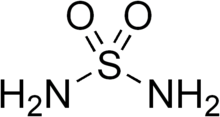
| |
| Names | |
|---|---|
| IUPAC name
Sulfamide
| |
| Other names
Sulfuryl amide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.029.330 |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| H4N2O2S | |
| Molar mass | 96.11 g/mol |
| Appearance | White orthorhombic plates |
| Melting point | 93 °C (199 °F; 366 K) |
| Boiling point | 250 °C (482 °F; 523 K) |
| Freely soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sulfamide is a chemical compound with the molecular structure H2NSO2NH2. Sulfamide is produced by the reaction of sulfuryl chloride with ammonia.
Sulfamide functional group
In organic chemistry, the term sulfamide may also refer to the functional group which consists of at least one organic group attached to a nitrogen atom of sulfamide.
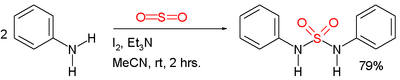
Symmetric sulfamides can be prepared directly from amines and sulfur dioxide gas[2]:
In this example, the reactants are aniline, triethylamine, and iodine. Sulfur dioxide is believed to be activated through a series of intermediates: Et3N-I+-I-, Et3N-I+-I3- and Et3N+-SO2-.
The sulfamide functional group is an increasingly common structural feature used in medicinal chemistry.[3]
See also
References
- ^ Merck Index, 11th Edition, 8894.
- ^ Sulfamides and sulfamide polymers directly from sulfur dioxide Alexander V. Leontiev, H. V. Rasika Dias and Dmitry M. Rudkevich Chem. Commun., 2006, 2887 - 2889, doi:10.1039/b605063h
- ^ Allen B Reitz, Garry R Smith, and Michael H Parker (2009). "The role of sulfamide derivatives in medicinal chemistry: a patent review (2006 – 2008)". Expert Opinion on Therapeutic Patents. 19 (10): 1449–1453. doi:10.1517/13543770903185920.
{{cite journal}}: CS1 maint: multiple names: authors list (link)