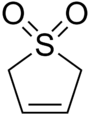
Sulfolene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2,5-Dihydrothiophene 1,1-dioxide
| |||
| Other names
Butadiene sulfone
3-Sulfolene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.000.964 | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C4H6O2S | |||
| Molar mass | 118.15 g·mol−1 | ||
| Melting point | 65 to 66 °C (149 to 151 °F; 338 to 339 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents.[2] The occasionally reported pungent smell[3] probably originates from adhering sulfur dioxide. The compound is used as a source of butadiene, but it is a versatile synthetic intermediate.In the laboratory it is used as a solid source of butadiene,[4] into which it decomposes by a reverse cycloaddition.[5]
Production
For the preparation of 3-sulfolene, liquid 1,3-butadiene is added in an autoclave at about −20 °C with an excess of liquid sulfur dioxide in the presence of small amounts of a phenolic polymerization inhibitor (e.g. hydroquinone or pyrogallol) and either allowed to stand at room temperature for eight days[6] or heated to about 130 °C for 30 minutes.[7]
After recrystallization from ethanol, the crystalline pure product is obtained in almost quantitative yield.
Reactions
Acid-base reactivity
The compound is unaffected by acids. It can even be recrystallized from conc. HNO3.[6]
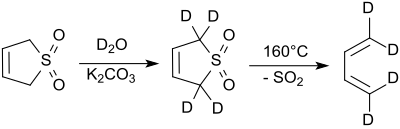
The protons in the 2- and 5-positions rapidly exchange with deuterium oxide under alkaline conditions.[8] Sodium cyanide catalyzes this reaction.[9].
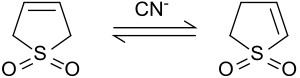
Isomerization to 2-sulfolene
Under basic conditions or when catalyzed by cyanide ions, 3-sulfolene isomerizes to a mixture of 2-sulfolene and 3-sulfolene. The ratio of 2-sulfolene and 3-sulfolene depends on the ratio of cyanide and sulfolene.[9]
At 50 °C an equilibrium mixture is obtained containing 42% 3-sulfolene and 58% 2-sulfolen.[10] The thermodynamically more stable 2-sulfolene can be isolated from the mixture of isomers as pure substance in the form of white plates (m.p. 48-49 °C) by heating for several days at 100 °C, because of the thermal decomposition of the 3-sulfolene at temperatures above 80 °C.[11]
Hydrogenation
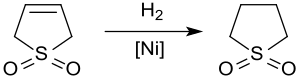
Catalytic hydrogenation yields sulfolane, a solvent used in the petrochemical industry for the extraction of aromatics from hydrocarbon streams. The hydrogenation of 3-sulfolene over Raney nickel at approx. 20 bar and 60 °C givessulfolane in yields of up to 65% only because of the poisoning of the catalyst by sulfur compounds.[12]
Halogenation
3-Sulfolene reacts in aqueous solution with bromine via an addition reaction to 3,4-dibromotetrohydrothiphene-1,1-dioxide, which can be dehydrobrominated to thiophene-1,1-dioxide with silver carbonate as demonstrated by H. Staudinger[6]. The reactive thiophene-1,1-dioxide, which is stable in solution only, is also accessible via the formation of 3,4-bis(dimethylamino)tetrahydrothiophene-1,1-dioxide and successive double quaternization with methyl iodide and Hofmann elimination with silver hydroxide.[11]
A less cumbersome two-step synthesis is the two-fold dehydrobromination of 3,4-dibromotetrohydrothiphene-1,1-dioxide with either powdered sodium hydroxide in tetrahydrofuran (THF)[13] or with ultrasonically dispersed metallic potassium.[14]
Diels-Alder reactions
3-sulfolene is valued as a stand-in for butadiene. The in situ production and immediate consumption of cis-1,3-butadiene largely avoids contact with the diene, which is a gas at room temperature.[15] One potential drawback, aside from expense, is that the evolved sulfur dioxide can cause side reactions with acid-sensitive substrates.
Above about 110 °C[2], 3-sulfolene decomposes to pure cis-1,3-butadiene and sulfur dioxide in a retro-cheletropic reaction.[6][16]
Diels-Alder reaction between 1,3-butadiene and dienophiles with low reactivity usually requires prolonged heating above 100 °C. This makes a procedure rather dangerous, if neat butadiene is used, and requiring special equipment for work under elevated pressure. Alternatively, butadiene can be generated in situ by thermal sulfur dioxide extrusion from sulfolene, in which case no buildup of butadiene pressure could be expected as the liberated diene is consumed in the cycloaddition, and therefore the equilibrium of the reversible extrusion reaction acts as an internal "safety valve".[17]
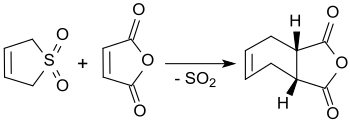
3-Sulfolene reacts with maleic anhydride in boiling xylene to cis-4-cyclohexene-1,2-dicarboxylic anhydride, obtaining yields of up to 90%.[18]
3-Sulfolene reacts also with dienophiles in trans configuration (such as diethyl fumarate) at 110 °C with SO2 elimination in 66–73% yield to the trans-4-cyclohexene-1,2-dicarboxylic diethyl ester.[19]
6,7-Dibromo-1,4-epoxy-1,4-dihydronaphthalene (6,7-Dibromonaphthalene-1,4-endoxide, accessible after debromination from 1,2,4,5-tetrabromobenzene using an equivalent of n-butyllithium and Diels-Alder reaction in furan in 70% yield[20]) reacts with 3-sulfolene in boiling xylene to a tricyclic adduct. This precursor yields, after treatment with perchloric acid, a dibromo dihydroanthracene which is dehydrogenated in the last step with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) to 2,3-dibromoanthracene.[21]
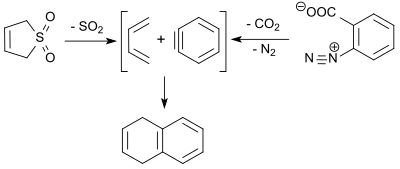
1,3-butadiene (formed in the retro-cheletrophic reaction of 3-sulfolene) reacts with dehydrobenzene (benzyne, obtained by thermal decomposition of benzenediazonium-2-carboxylate) in a Diels-Alder reaction in 9% yield to give 1,4-dihydronaphthalene.[22]
2- and 3-Sulfolenes as a dienophile
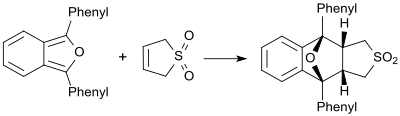
In the presence of very reactive dienes (for example 1,3-diphenylisobenzofuran) butadienesulfone behaves as a dienophile and forms the corresponding Diels-Alder adduct.[23]
As early as 1938, Kurt Alder and co-workers reported Diels-Alder adducts from the isomeric 2-sulfolene with 1,3-butadiene and 2-sulfolene with cyclopentadiene.[24]
Other cycloadditions
Thiophene-1,1-dioxide reacts via a [4+6] cycloaddition with 6-dimethylaminofulvene[25] for the synthesis of the aromatic hydrocarbon azulene.[13] The overall yield of azulen is 33%.[26]
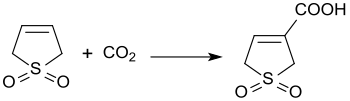
The base-catalyzed reaction of 3-sulfolene with carbon dioxide at 3 bar pressure produces 3-sulfolene-3-carboxylic acid in 45% yield.[27]
With diazomethane, 3-sulfolene forms in a 1,3-dipolar cycloaddition a fused, five-membered ring system in high yield.[28]
Polymerization
In 1935, H. Staudinger and co-workers found that in the reaction of butadiene and SO2 at room temperature, in addition to crystalline 3-sulfolene (89% yield), an amorphous solid polymer is formed in small amounts. By free-radical polymerization of 3-sulfolene in peroxide-containing diethyl ether, they obtained up to 50% insoluble high-molecular-weight poly-sulfolenes,[16] which were also stable in concentrated nitric and sulfuric acid.
In subsequent investigations, 3-sulfols could not be polymerized ionically or radically, but above 100 °C and with the radical initiator azobis(isobutyronitrile) (AIBN), to form polybutadienesulfone.[29] 3-sulfoles do not copolymerize with vinyl compounds, though. On the contrary, 2-sulfolene does not homopolymerize, but forms copolymers with vinyl compounds, e.g. acrylonitrile and vinyl acetate.
3-Sulfolene as a recyclable solvent
The reversibility of the formation of 3-sulfoles and its decomposition into the starting materials 1,3-butadiene and sulfur dioxide suggests the use of butadienesulfone as a recyclable aprotic dipolar solvent, in replacement for dimethyl sulfoxide (DMSO), which is often used but difficult to separate and poorly reusable.[30] As a model reaction, the reaction of benzyl azide with 4-toluenesulfonic acid cyanide forming 1-benzyl-5-(4-toluenesulfonyl)tetrazole was investigated. The formation of the tetrazole can also be carried out as a one-pot reaction without the isolation of the benzyl azide with 72% overall yield.
After the reaction, the solvent 3-sulfolene is decomposed at 135 °C and the volatile butadiene (m.p. −4.5 °C) and sulfur dioxide (m.p. −10.1 °C) are deposited in a cooling trap at −76 °C charged with excess sulfur dioxide. After the addition of hydroquinone as polymerization inhibition, 3-sulfoles is formed again quantitatively upon heating to room temperature. It appears questionable though, if 3-sulfolene with a useful liquid phase range of only 64 to a maximum of about 100 °C can be used as DMSO substitutes (easy handling, low cost, environmental compatibility) in industrial practice.
Uses
Aside from its synthetic versatility (see above), sulfolene is used as an additive in electrochemical fluorination. It can increase the yield of perfluorooctanesulfonyl fluoride by about 70%.[31] It is "highly soluble in anhydrous HF and increases the conductivity of the electrolyte solution".[31] In this application, it undergoes a ring opening and is fluorinated to form perfluorobutanesulfonyl fluoride.
References
- ^ Sulfolene at Sigma-Aldrich
- ^ a b J.M. McIntosh (2001), "3-Sulfolene" (in German), e-EROS Encyclopedia of Reagents for Organic Synthesis, doi:10.1002/047084289X.rs130
- ^ "Datasheet 3-sulfolene for synthesis (PDF)". Retrieved 2017-12-12.
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help) - ^ Sample, Thomas E.; Hatch, Lewis F (Jan 1968). "3-Sulfolene: A butadiene source for a Diels-Alder synthesis". Journal of Chemical Education. 45 (1): 55. doi:10.1021/ed045p55.
- ^ Leo Paquette (ed), Encyclopedia of Reagents for Organic Synthesis, p. 4678 ff
- ^ a b c d DE 506839, H. Staudinger, "Verfahren zur Darstellung von monomolekularen Reaktionsprodukten von ungesättigten Kohlenwasserstoffen der Butadienreihe mit Schwefeldioxyd"
- ^ Houben-Weyl (1955). Volume IX: Sulfur, Selenium, Tellurium Compounds. Methods of Organic Chemistry (4th ed.). Stuttgart: Georg Thieme Verlag. p. 237. ISBN 978-3-13-208104-8.
- ^ J. Leonard; A. B. Hague; J. A. Knight (1998). "6. Preparation of substituted 3-sulfolenes and their use as precursors for Diels-Alder dienes". Organosulfur Chemistry. Vol. 2 (4th ed.). San Diego: Academic Press, Inc. p. 241. ISBN 0-12-543562-2.
- ^ a b US 4187231, R. L. Cobb, "Cyanide-catalyzed isomerization and deuterium exchange with alpha- and beta-sulfolenes"
- ^ C. D. Broaddus (1968). "Equilibria and base-catalyzed exchange of substituted olefins". Accounts of Chemical Research. 1 (8): 231–238. doi:10.1021/ar50008a002.
- ^ a b W. J. Bailey; E. W. Cummins (1954). "Cyclic dienes. III. The synthesis of thiophene-1,1-dioxide". Journal of the American Chemical Society. 76 (7): 1932–1936. doi:10.1021/ja01636a058.
- ^ US 4286099, M. E. Nash, E. E. Huxley, "Sulfolene hydrogenation"
- ^ a b D. M. Lemal; G. D. Goldman (1988). "Synthesis of azulene, a blue hydrocarbon". Journal of Chemical Education. 65 (10): 923. doi:10.1021/ed065p923.
- ^ T.-S. Chou; M.-M. Chen (1987). "Chemoselective reactions of ultrasonically dispersed potassium with some brominated hydrothiophene-S,S-dioxides". Heterocycles. 26 (11): 2829–2834. doi:10.3987/R-1987-11-2829.
- ^ Leo Paquette (ed), Encyclopedia of Reagents for Organic Synthesis, p. 4678 ff
- ^ a b H. Staudinger; B. Ritzenthaler (1935). "Über hochmolekulare Verbindungen, 104. Mitteil.: Über die Anlagerung von Schwefeldioxyd an Äthylen-Derivate". Chemische Berichte (in German). 68 (3): 455–471. doi:10.1002/cber.19350680317.
- ^ M. A. Filatov; S. Baluschev; I. Z. Ilieva; V. Enkelmann; T. Miteva; K. Landfester; S. E. Aleshchenkov; A. V. Cheprakov (2012). "Tetraaryltetraanthra[2,3]porphyrins: Synthesis, Structure, and Optical Properties". The Journal of Organic Chemistry. 77 (24): 11119–11131. doi:10.1021/jo302135q.
- ^ T. E. Sample, Jr., L. F. Hatch (1968). "3-Sulfolene: a butadiene source for a Diels-Alder synthesis: an undergraduate laboratory experiment". Journal of Chemical Education. 45 (1): 55. doi:10.1021/ed045p55.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Diethyl trans-Δ4-tetrahydrophthalate". Organic Syntheses. 50. doi:10.15227/orgsyn.050.0043.
- ^ H. Hart, A. Bashir-Hashemi, J. Luo, M.A. Meador (1986). "Iptycenes: Extended triptycenes". Tetrahedron. 42 (6): 1641–1654. doi:10.1016/S0040-4020(01)87581-5.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ C.-T. Lin, T.-C. Chou (1988). "Synthesis of 2,3-dibromoanthracene". Synthesis. 1988 (8): 628–630. doi:10.1055/s-1988-27659.
- ^ L. F. Hatch, D. Peter (1968). "Reaction of benzyne with butadiene". Chemical Communications. 23: 1499. doi:10.1039/C19680001499.
- ^ M. P. Cava, J. P. VanMeter (1969). "Condensed cyclobutane aromatic compounds. XXX. Synthesis of some unusual 2,3-naphthoquinonoid heterocycles. A synthetic route to derivatives of naphtho[2,3-b]biphenylene and anthra[b]cyclobutene". The Journal of Organic Chemistry. 34 (3): 538–545. doi:10.1021/jo01255a012.
- ^ K. Alder; H. F. Rickert; E. Windemuth (1938). "Zur Kenntnis der Dien-Synthese, X. Mitteil.: Über die Dien-Synthese mit α, β-ungesättigten Nitrokörpern, Sulfonen und Thio-Äthern". Chemische Berichte (in German). 71 (12): 2451–2461. doi:10.1002/cber.19380711206.
- ^ "6-(Dimethylamino)fulvene". Organic Syntheses. 47. doi:10.15227/orgsyn.047.0052.
- ^ D. Copland; D. Leaver; W. B. Menzies (1977). "A new and convenient synthesis of azulenes from 6-N,N-dimethylaminofulvene and thiophene-1,1-dioxides". Tetrahedron Letters. 18 (7): 639–640. doi:10.1016/S0040-4039(01)92713-3.
- ^ G. S. Andrade; et al. (2003). "The one-pot synthesis and Diels-Alder reactivity of 2,5-dihydrothiophene-1,1-dioxide-3carboxylic acid". Synthetic Communications. 33 (20): 3643–3650. doi:10.1081/SCC-120024845.
- ^ M. E. Brant; J. E. Wulff (2016). "3-Sulfolenes and their derivatives: Synthesis and applications". Synthesis. 48 (01): 1–17. doi:10.1055/s-0035-1560351.
- ^ E. J. Goethals (1967). "On the polymerization and copolymerization of sulfolenes". Macromolecular Chemistry and Physics. 109 (1): 132–142. doi:10.1002/macp.1967.021090113.
- ^ Y. Huang; et al. (2015). "Butadiene sulfone as 'volatile', recyclable dipolar, aprotic solvent for conducting substitution and cycloaddition reactions". Sustainable Chemical Processes. 3 (13). doi:10.1186/s40508-015-0040-7.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Lehmler HJ (March 2005). "Synthesis of environmentally relevant fluorinated surfactants—a review". Chemosphere. 58 (11): 1471–96. doi:10.1016/j.chemosphere.2004.11.078. PMID 15694468.