Surface-area-to-volume ratio
The surface-area-to-volume ratio, also called the surface-to-volume ratio and variously denoted sa/vol or SA:V, is the amount of surface area per unit volume of an object or collection of objects. In chemical reactions involving a solid material, the surface area to volume ratio is an important factor for the reactivity, that is, the rate at which the chemical reaction will proceed.
For a given volume, the object with the smallest surface area (and therefore with the smallest SA:V) is the sphere, a consequence of the isoperimetric inequality in 3 dimensions. By contrast, objects with tiny spikes will have very large surface area for a given volume.
Dimension
The surface-area-to-volume ratio has physical dimension L−1 (inverse length) and is therefore expressed in units of inverse distance. As an example, a cube with sides of length 1 cm will have a surface area of 6 cm2 and a volume of 1 cm3. The surface to volume ratio for this cube is thus
- .
For a given shape, SA:V is inversely proportional to size. A cube 2 cm on a side has a ratio of 3 cm−1, half that of a cube 1 cm on a side. Conversely, preserving SA:V as size increases requires changing to a less compact shape.
Physical chemistry
Materials with high surface area to volume ratio (e.g. very small diameter, very porous, or otherwise not compact) react at much faster rates than monolithic materials, because more surface is available to react. Examples include grain dust; while grain isn't typically flammable, grain dust is explosive. Finely ground salt dissolves much more quickly than coarse salt.
High surface area to volume ratio provides a strong "driving force" to speed up thermodynamic processes that minimize free energy.
Biology
The ratio between the surface area and volume of cells and organisms has an enormous impact on their biology, including their physiology and behavior. For example, many aquatic microorganisms have increased surface area to increase their drag in the water. This reduces their rate of sink and allows them to remain near the surface with less energy expenditure.[citation needed]
An increased surface area to volume ratio also means increased exposure to the environment. The finely-branched appendages of filter feeders such as krill provide a large surface area to sift the water for food.[1]
Individual organs like the lung have numerous internal branchings that increase the surface area; in the case of the lung, the large surface supports gas exchange, bringing oxygen into the blood and releasing carbon dioxide from the blood.[2][3] Similarly, the small intestine has a finely wrinkled internal surface, allowing the body to absorb nutrients efficiently.[4]
Cells can achieve a high surface area to volume ratio with an elaborately convoluted surface, like the microvilli lining the small intestine.[5]
Increased surface area can also lead to biological problems. More contact with the environment through the surface of a cell or an organ (relative to its volume) increases loss of water and dissolved substances. High surface area to volume ratios also present problems of temperature control in unfavorable environments.[citation needed]
The surface to volume ratios of organisms of different sizes also leads to some biological rules such as Bergmann's rule[6][7][8] and gigantothermy.[9]
Fire spread
In the context of wildfires, the ratio of the surface area of a solid fuel to its volume is an important measurement. Fire spread behavior is frequently correlated to the surface-area-to-volume ratio of the fuel (e.g. leaves and branches). The higher its value, the faster a particle responds to changes in environmental conditions, such as temperature or moisture. Higher values are also correlated to shorter fuel ignition times, and hence faster fire spread rates.
Planetary cooling
A body of icy or rocky material in outer space may, if it can build and retain sufficient heat, develop a differentiated interior and alter its surface through volcanic or tectonic activity. The length of time through which a planetary body can maintain surface-altering activity depends on how well it retains heat, and this is governed by its surface area-to-volume ratio. For Vesta (r=263 km), the ratio is so high that astronomers were surprised to find that it did differentiate and have brief volcanic activity. The moon, Mercury and Mars have radii in the low thousands of kilometers; all three retained heat well enough to be thoroughly differentiated although after a billion years or so they became too cool to show anything more than very localized and infrequent volcanic activity, of which none is evident at present. Venus and Earth (r>6,000 km) have sufficiently low surface area-to-volume ratios (roughly half that of Mars and much lower than all other known rocky bodies) so that their heat loss is minimal.[10]
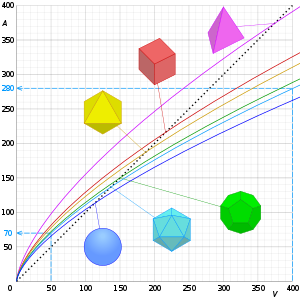
Mathematical examples
| Shape | Characteristic Length | Surface Area | Volume | SA/V ratio | SA/V ratio for unit volume | |
|---|---|---|---|---|---|---|
| Tetrahedron | 
|
side | 7.21 | |||
| Cube | 
|
side | 6 | |||
| Octahedron | 
|
side | 5.72 | |||
| Dodecahedron | 
|
side | 5.31 | |||
| Capsule | 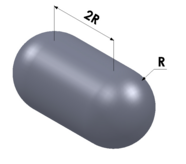
|
radius (R) | 5.251 | |||
| Icosahedron | 
|
side | 5.148 | |||
| Sphere | 
|
radius | 4.83515 |
| side of cube | side2 | Area of side | 6*side2 | Area of Cube's Surface | side3 | Volume | Ratio of Surface Area to Volume |
|---|---|---|---|---|---|---|---|
| 2 | 2x2 | 4 | 6x2x2 | 24 | 2x2x2 | 8 | 3:1 |
| 4 | 4x4 | 16 | 6x4x4 | 96 | 4x4x4 | 64 | 3:2 |
| 6 | 6x6 | 36 | 6x6x6 | 216 | 6x6x6 | 216 | 3:3 |
| 8 | 8x8 | 64 | 6x8x8 | 384 | 8x8x8 | 512 | 3:4 |
| 12 | 12x12 | 144 | 6x12x12 | 864 | 12x12x12 | 1728 | 3:6 |
| 20 | 20x20 | 400 | 6x20x20 | 2400 | 20x20x20 | 8000 | 3:10 |
| 50 | 50x50 | 2500 | 6x50x50 | 15000 | 50x50x50 | 125000 | 3:25 |
| 1000 | 1000x1000 | 1000000 | 6x1000x1000 | 6000000 | 1000x1000x1000 | 1000000000 | 3:500 |
See also
References
- Schmidt-Nielsen, Knut (1984). Scaling: Why is Animal Size so Important?. New York, NY: Cambridge University Press. ISBN 978-0-521-26657-4. OCLC 10697247.
- Vogel, Steven (1988). Life's Devices: The Physical World of Animals and Plants. Princeton, NJ: Princeton University Press. ISBN 978-0-691-08504-3. OCLC 18070616.
- Specific
- ^ Kils, U.: Swimming and feeding of Antarctic Krill, Euphausia superba - some outstanding energetics and dynamics - some unique morphological details. In Berichte zur Polarforschung, Alfred Wegener Institute for Polar and Marine Research, Special Issue 4 (1983): "On the biology of Krill Euphausia superba", Proceedings of the Seminar and Report of Krill Ecology Group, Editor S. B. Schnack, 130-155 and title page image.
- ^ Tortora, Gerard J.; Anagnostakos, Nicholas P. (1987). Principles of anatomy and physiology (Fifth ed.). New York: Harper & Row, Publishers. pp. 556–582. ISBN 0-06-350729-3.
- ^ Williams, Peter L; Warwick, Roger; Dyson, Mary; Bannister, Lawrence H. (1989). Gray’s Anatomy (Thirty-seventh ed.). Edinburgh: Churchill Livingstone. pp. 1278–1282. ISBN 0443 041776.
- ^ Romer, Alfred Sherwood; Parsons, Thomas S. (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 349–353. ISBN 0-03-910284-X.
- ^ Krause J. William (July 2005). Krause's Essential Human Histology for Medical Students. Universal-Publishers. pp. 37–. ISBN 978-1-58112-468-2. Retrieved 25 November 2010.
- ^ Meiri, S.; Dayan, T. (2003-03-20). "On the validity of Bergmann's rule". Journal of Biogeography. 30 (3): 331–351. doi:10.1046/j.1365-2699.2003.00837.x.
{{cite journal}}: Unknown parameter|subscription=ignored (|url-access=suggested) (help) - ^ Ashton, Kyle G.; Tracy, Mark C.; Queiroz, Alan de (October 2000). "Is Bergmann's Rule Valid for Mammals?". The American Naturalist. 156 (4): 390–415. doi:10.1086/303400. JSTOR 10.1086/303400.
- ^ Millien, Virginie; Lyons, S. Kathleen; Olson, Link; et al. (May 23, 2006). "Ecotypic variation in the context of global climate change: Revisiting the rules". Ecology Letters. 9 (7): 853–869. doi:10.1111/j.1461-0248.2006.00928.x.
- ^ Fitzpatrick, Katie (2005). "Gigantothermy". Davidson College. Archived from the original on 2012-06-30. Retrieved 2011-12-21.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ^ http://www.astro.uvic.ca/~venn/A201/maths.6.planetary_cooling.pdf
External links
- Sizes of Organisms: The Surface Area:Volume Ratio
- National Wildfire Coordinating Group: Surface Area to Volume Ratio
- Previous link not working, references are in this document, PDF























