Tetrakis(dimethylamino)ethylene

| |
| Names | |
|---|---|
| Preferred IUPAC name
N1,N1,N′1,N′1,N2,N2,N′2,N′2-Octamethylethene-1,1,2,2-tetramine | |
| Other names
Octamethyl-ethenetetramine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.012.398 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H24N4 | |
| Molar mass | 200.330 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.861 g/cm3 (25 °C) |
| Melting point | −4 °C (25 °F; 269 K) |
| Boiling point | 59 °C (0.9 mm Hg) |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H226, H314 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P370+P378, P403+P235, P405, P501 | |
| Flash point | 53 °C (127 °F; 326 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetrakis(dimethylamino)ethylene (TDAE) is an organic compound with the formula [C(NMe2)4]2 (where Me = CH3). A colorless liquid, this compound is classified as an enamine. Primary and secondary enamines tend to isomerize, but tertiary enamines are kinetically stable. The unusual feature of TDAE is that it is a tetra-enamine. The pi-donating tendency of the amine groups strongly modifies the properties of the molecule, which does exhibit properties of a typical alkene.[1]
Reactions
TDAE reacts with oxygen in a chemiluminscent reaction to give tetramethylurea[2][3]

TDAE is an electron donor with E = 1.06 v vs Fc+/0.[4] It forms a charge transfer salt with buckminsterfullerene:[5]
- C2(N(CH3)2)4 + C60 → [C2(N(CH3)2)4+][C60−]
Oxidation affords a dication.
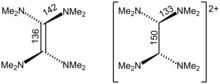
Structure
Crystallographic analysis show that TDAE is a highly distorted alkene, the dihedral angle for the two N2C termini is 28″. The C=C distance is alkene-like, 135 pm. The nearly isostructural tetraisopropylethylene also has a C=C distance of 135 pm, but its C6 core is planar. In contrast, [TDAE]2+ is an alkane with multi-C-N bonds.
References
- ^ David M. Lemal (1968). "Tetraaminoethylenes". In Saul Patai (ed.). The Amino Group. pp. 701–748. doi:10.1002/9780470771082. ISBN 9780470771082.
- ^ H.E. Winberg; J. R. Downing; D. D. Coffman (1965). "The Chemiluminescence of Tetrakis(dimethylamino)ethylene". J. Am. Chem. Soc. 87 (9): 2054–2055. doi:10.1021/ja01087a039.
- ^ "Chemilumineszenz von TDAE" (in German). illumina-chemie.de. 2014-08-08. Retrieved 2016-08-22.
- ^ Kuroboshi, Manabu; Waki, Yoko; Tanaka, Hideo (2003). "Palladium-Catalyzed Tetrakis(dimethylamino)ethylene-Promoted Reductive Coupling of Aryl Halides". The Journal of Organic Chemistry. 68 (10): 3938–3942. doi:10.1021/jo0207473. PMID 12737575.
- ^ Allemand PM, Khemani KC, Koch A, et al. (1991). "Organic Molecular Soft Ferromagnetism in a Fullerene". Science. 253 (5017): 301–302. Bibcode:1991Sci...253..301A. doi:10.1126/science.253.5017.301. PMID 17794696. S2CID 19561675.
- ^ Bock, Hans; Borrmann, Horst; Havlas, Zdenek; et al. (1991). "Tetrakis(dimethylamino)ethene: An Extremely Electron-Rich Molecule with Unusual Structure both in the Crystal and in the Gas Phase". Angewandte Chemie International Edition in English. 30 (12): 1678–1681. doi:10.1002/anie.199116781.
