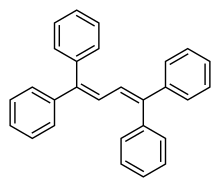
Tetraphenyl butadiene
| |
| Names | |
|---|---|
| IUPAC name
1,1,4,4-Tetraphenyl-1,3-butadiene
| |
| Identifiers | |
| ChemSpider | |
| ECHA InfoCard | 100.014.468 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C28H22 | |
| Molar mass | 358.475 g/mol |
| Appearance | white to yellow white needles |
| Density | 1.079g/cm3 |
| Melting point | 203.5 °C (398.3 °F; 476.6 K) |
| Boiling point | 556.1 °C (1,033.0 °F; 829.2 K) at 760 mmHg |
| Solubility | soluble in ethanol, benzene, chloroform, acetic acid[1] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 289 °C (552 °F; 562 K) |
| Safety data sheet (SDS) | Sigma-Aldrich |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetraphenyl butadiene (IUPAC name: 1,1,4,4-tetraphenyl-1,3-butadiene, abbreviated TPB) is an organic chemical compound used as an electroluminescent dye. It glows blue with an emission spectrum peak wavelength at 430 nm,[2] which makes it useful as a wavelength shifter.[3][4]
References
- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 3–526. ISBN 0-8493-0594-2.
- ^ W. M. Burton and B. A. Powell, "Fluorescence of Theraphenyl-Butadiene in the Vacuum Ultraviolet", Applied Optics, Vol. 12, Issue 1, pp. 87-89 (1973), doi:10.1364/AO.12.000087.
- ^ Wise, Donald Lee; Gary E. Wnek; Debra J. Trantolo; Thomas M. Cooper; Joseph D. Gresser (1998). Photonic Polymer Systems. CRC Press. p. 250. ISBN 978-0-8247-0152-9. Retrieved 2009-06-02.
- ^ Wernick, Miles N.; John N. Aarsvold (2004). Emission Tomography. Academic Press. p. 374. ISBN 978-0-12-744482-6. Retrieved 2009-06-02.

