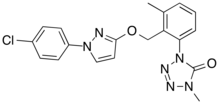
Metyltetraprole
| |
| Names | |
|---|---|
| IUPAC name
1-[2-[[1-(4-chlorophenyl)pyrazol-3-yl]oxymethyl]-3-methylphenyl]-4-methyltetrazol-5-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H17ClN6O2 | |
| Molar mass | 396.84 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Metyltetraprole is a quinone outside inhibitor fungicide sold under the brand name Pavecto by its inventor, Sumitomo Chemical.[1] It is the only tetrazolinone fungicide and the only one in the Fungicide Resistance Action Committee's subgroup 11A.[2]
Development
[edit]Metyltetraprole was developed specifically to find an a.i. with the same mode of action (a QoI) but with sufficiently different chemistry as to avoid "critical" QoI resistance increasing around the world.[3]
Target pathogens
[edit]Metyltetraprole is highly effective against Alternaria triticina.[1]
Resistance
[edit]Developed because of increasing resistance to the main group of QoIs. See §Development above.
Cross-resistance
[edit]It does not suffer cross-resistance with the resistance against 11 conferred by the cytochrome b mutation G143A. Cross-resistance against F129L is unassessed.[2]
Binding Mode
[edit]The structure of the tetrazolinone pharmacophore is very similar to the triazolone pharmacophore of an inhibitor developed by AgoEva, for which the binding mode has been elucidated in the structure deposited as 3L73 in the protein databank.
References
[edit]- ^ a b Umetsu, Noriharu; Shirai, Yuichi (2020-05-20). "Development of novel pesticides in the 21st century". Journal of Pesticide Science. 45 (2). Pesticide Science Society of Japan: 54–74. doi:10.1584/jpestics.d20-201. eISSN 1349-0923. ISSN 1348-589X. PMC 7581488. PMID 33132734. ISSN-L 0385-1559
- ^ a b FRAC (Fungicide Resistance Action Committee) (March 2021). "FRAC Code List ©*2021: Fungal control agents sorted by cross resistance pattern and mode of action (including coding for FRAC Groups on product labels)" (PDF). pp. 1–17.
- ^ Matsuzaki, Yuichi; Yoshimoto, Yuya; Arimori, Sadayuki; Kiguchi, So; Harada, Toshiyuki; Iwahashi, Fukumatsu (2020). "Discovery of metyltetraprole: Identification of tetrazolinone pharmacophore to overcome QoI resistance". Bioorganic & Medicinal Chemistry. 28 (1). Elsevier: 115211. doi:10.1016/j.bmc.2019.115211. ISSN 0968-0896. PMID 31753801.
