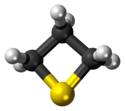
Thietane
Appearance
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Thietane
| |||
| Other names
Thiacyclobutane
Trimethylene sulfide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 102383 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.469 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UN number | 1993 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H6S | |||
| Molar mass | 74.14 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Sulfurous | ||
| Density | 1.028 g cm−3 | ||
| Boiling point | 94 to 95 °C (201 to 203 °F; 367 to 368 K) | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H225, H302 | |||
| P210 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | -11(9) °C | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Thietane is a heterocyclic compound containing a saturated four-membered ring with three carbon atoms and one sulfur atom.[1][2]
Thietane, and its derivative 2-propylthietane, are strong-smelling mouse alarm pheromones and predator scent analogues.[3][4] Both the mouse and human olfactory receptors MOR244-3 and OR2T11, respectively, were found to respond to thietane in the presence of copper.[5]
References
- ^ Leśniak, S; Lewkowski, J; Kudelska, W; Zając, A (2008). "Thietanes and Thietes: Monocyclic". Comprehensive Heterocyclic Chemistry III. 2.07: 389–428. doi:10.1016/B978-008044992-0.00207-8.
{{cite journal}}: Cite has empty unknown parameter:|1=(help) - ^ Block, E; DeWang, M (1996). "Thietanes and Thietes: Monocyclic". Comprehensive Heterocyclic Chemistry II. 1.24: 773–802. doi:10.1016/B978-008096518-5.00024-1.
{{cite journal}}: Cite has empty unknown parameter:|1=(help) - ^ Sievert, Thorbjörn; Laska, Matthias (2016). "Behavioral responses of CD-1 mice to six predator odor components". Chem. Senses. 41 (5): 399–406. doi:10.1093/chemse/bjw015. PMID 26892309.
{{cite journal}}: Cite has empty unknown parameter:|1=(help) - ^ Brechbuhl, J; Moine, F; Klaey, M; Nenniger-Tosato, M; Hurni, N; Sporkert, F; Giroud, C; Broillet, MC (2013). "Mouse alarm pheromone shares structural similarity with predator scents". Proc. Natl. Acad. Sci. U. S. A. 110 (12): 4762–4767. doi:10.1073/pnas.1214249110. PMID 23487748.
{{cite journal}}: Cite has empty unknown parameter:|1=(help) - ^ Li, Shengju; Ahmed, Lucky; Zhang, Ruina; Pan, Yi; Matsunami, Hiroaki; Burger, Jessica L; Block, Eric; Batista, Victor S; Zhuang, Hanyi (2016). "Smelling sulfur: Copper and silver regulate the response of human odorant receptor OR2T11 to low molecular weight thiols". Journal of the American Chemical Society. in press. doi:10.1021/jacs.6b06983. PMID 27659093.
{{cite journal}}: Cite has empty unknown parameter:|1=(help)