Thiocyanate

| |
| Names | |
|---|---|
| IUPAC name
thiocyanate
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| Properties | |
| CNS- | |
| Molar mass | 58.0824 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thiocyanate (also known as sulphocyanate or thiocyanide or rhodanide) is the anion [SCN]− and the conjugate base of thiocyanic acid. Common compounds include the colourless salts potassium thiocyanate and sodium thiocyanate. Organic compounds containing the functional group SCN are also called thiocyanates. Mercury(II) thiocyanate was formerly used in pyrotechnics.
Thiocyanate is analogous to the cyanate ion, [OCN]−, where in oxygen is replaced by sulfur. [SCN]− is one of the pseudohalides, due to the similarity of its reactions to that of halide ions. Thiocyanate was formerly known as rhodanide (from a Greek word for rose) because of the red colour of its complexes with iron. Thiocyanate is produced by the reaction of elemental sulfur or thiosulfate with cyanide:
- 8 CN− + S8 → 8 SCN−
- CN− + S2O32− → SCN− + SO32−
The latter reaction is catalyzed by the enzyme sulfotransferase known as rhodanase and may be relevant to detoxification of cyanide in the body.
Structure, bonding and coordination chemistry
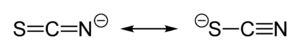
Thiocyanate shares its negative charge approximately equally between sulfur and nitrogen. Consequently, thiocyanate can act as a nucleophile at either sulfur or nitrogen — it is an ambidentate ligand. [SCN]− can also bridge two (M−SCN−M) or even three metals (>SCN− or −SCN<). Experimental evidence leads to the general conclusion that class a metals (hard acids) tend to form N-bonded thiocyanate complexes, whereas class b metals (soft acids) tend to form S-bonded thiocyanate complexes. Other factors, e.g. kinetics and solubility, are sometimes involved, and linkage isomerism can occur, for example [Co(NH3)5(NCS)]Cl2 and [Co(NH3)5(SCN)]Cl2[1].
Organic thiocyanates
Organic and transition metal derivatives of the thiocyanate ion can exist as "linkage isomers." In thiocyanates, the organic group or metal is attached to sulfur: R−S−C≡N has a S-C single bond and a C-N triple bond [2]. In isothiocyanates, the substituent is attached to nitrogen: R−N=C=S has a S-C double bond and a C-N double bond

Organic thiocyanates are hydrolyzed to thiocarbamates in the Riemschneider thiocarbamate synthesis.
Test for iron(III)
If [SCN]− is added to a solution containing iron(III) ions (Fe3+), a blood red solution is formed due to the formation of [Fe(NCS)(H2O)5]2+.

References
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. p. 326
- ^ Guy, R. G. "Syntheses and Preparative Applications of Thiocyanates" in "Chemistry of Cyanates and Their Derivatives," vol II. Patai, S., (Editor), John Wiley, 1977. New York
