Thiocyanate

| |
| Names | |
|---|---|
| IUPAC name
thiocyanate
| |
| Other names
sulphocyanate, thiocyanide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| Properties | |
| CNS- | |
| Molar mass | 58.0824 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thiocyanate (also known as rhodanide) is the anion [SCN]−. It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Organic compounds containing the functional group SCN are also called thiocyanates. Mercury(II) thiocyanate was formerly used in pyrotechnics.
Thiocyanate is analogous to the cyanate ion, [OCN]−, wherein oxygen is replaced by sulfur. [SCN]− is one of the pseudohalides, due to the similarity of its reactions to that of halide ions. Thiocyanate used to be known as rhodanide (from a Greek word for rose) because of the red colour of its complexes with iron. Thiocyanate is produced by the reaction of elemental sulfur or thiosulfate with cyanide:
- 8 CN− + S8 → 8 SCN−
- CN− + S2O32− → SCN− + SO32−
The second reaction is catalyzed by the enzyme sulfotransferase known as rhodanase and may be relevant to detoxification of cyanide in the body.
Structure, bonding and coordination chemistry
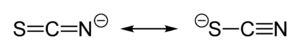
Thiocyanate shares its negative charge approximately equally between sulfur and nitrogen. As a consequence, thiocyanate can act as a nucleophile at either sulfur or nitrogen — it is an ambidentate ligand. [SCN]− can also bridge two (M−SCN−M) or even three metals (>SCN− or −SCN<). Experimental evidence leads to the general conclusion that class a metals (hard acids) tend to form N-bonded thiocyanate complexes, whereas class b metals (soft acids) tend to form S-bonded thiocyanate complexes. Other factors, e.g. kinetics and solubility, are sometimes involved, and linkage isomerism can occur, for example [Co(NH3)5(NCS)]Cl2 and [Co(NH3)5(SCN)]Cl2[1].
Organic thiocyanates
Organic and transition metal derivatives of the thiocyanate ion can exist as "linkage isomers." In thiocyanates, the organic group (or metal ion) is attached to sulfur: R−S−C≡N has a S-C single bond and a C-N triple bond [2]. In isothiocyanates, the substituent is attached to nitrogen: R−N=C=S has a S-C double bond and a C-N double bond:

Organic thiocyanates are hydrolyzed to thiocarbamates in the Riemschneider thiocarbamate synthesis.
Test for iron(III)
If [SCN]− is added to a solution containing iron (III) ions (Fe3+), a blood red solution is formed due to the formation of [Fe(NCS)(H2O)5]2+.

Biological chemistry of thiocyanate in medicine
Thiocyanate[3] is known to be an important part in the biosynthesis of hypothiocyanite by a lactoperoxidase.[4][5][6] Thus the complete absence of thiocyanate[7] or reducted thiocyanate[8] in the human body, (e.g., cystic fibrosis) is of high importance in the human host defense system.[9][10]
References
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. p. 326
- ^ Guy, R. G. "Syntheses and Preparative Applications of Thiocyanates" in "Chemistry of Cyanates and Their Derivatives," vol II. Patai, S., (Editor), John Wiley, 1977. New York
- ^ Pedemonte N, Caci E, Sondo E, Caputo A, Rhoden K, Pfeffer U, Di Candia M, Bandettini R, Ravazzolo R, Zegarra-Moran O, Galietta LJ (2007). "Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels". J. Immunol. 178 (8): 5144–53. PMID 17404297.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Conner GE, Wijkstrom-Frei C, Randell SH, Fernandez VE, Salathe M (2007). "The lactoperoxidase system links anion transport to host defense in cystic fibrosis". FEBS Lett. 581 (2): 271–8. doi:10.1016/j.febslet.2006.12.025. PMC 1851694. PMID 17204267.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ White WE, Pruitt KM, Mansson-Rahemtulla B (1983). "Peroxidase-thiocyanate-peroxide antibacterial system does not damage DNA". Antimicrob. Agents Chemother. 23 (2): 267–72. PMC 186035. PMID 6340603.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Thomas EL, Aune TM (1978). "Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action". Infect. Immun. 20 (2): 456–63. PMC 421877. PMID 352945.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Childers M, Eckel G, Himmel A, Caldwell J (2007). "A new model of cystic fibrosis pathology: lack of transport of glutathione and its thiocyanate conjugates". Med. Hypotheses. 68 (1): 101–12. doi:10.1016/j.mehy.2006.06.020. PMID 16934416.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Minarowski Ł, Sands D, Minarowska A, Karwowska A, Sulewska A, Gacko M, Chyczewska E (2008). "Thiocyanate concentration in saliva of cystic fibrosis patients". Folia Histochem. Cytobiol. 46 (2): 245–6. doi:10.2478/v10042-008-0037-0. PMID 18519245.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Nauseef WM, Dupuy C, Bánfi B (2007). "A novel host defense system of airways is defective in cystic fibrosis". Am. J. Respir. Crit. Care Med. 175 (2): 174–83. doi:10.1164/rccm.200607-1029OC. PMC 2720149. PMID 17082494.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Xu Y, Szép S, Lu Z (2009). "The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases". Proc. Natl. Acad. Sci. U.S.A. 106 (48): 20515–9. doi:10.1073/pnas.0911412106. PMC 2777967. PMID 19918082.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
