Thioureas
Thioureas are members of a family of organosulfur compounds with the formula SC(NR2)2. The parent member of this class of compounds is thiourea SC(NH2)2. The thiourea functional group has a planar CSN2 core.

Structure and bonding
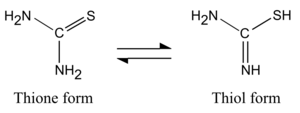
Thioureas have planar N2CS core. The C=S bond distance is near 1.71 Å, which is 0.1 Å longer than in normal ketones. The C-N distances are short.[1] Thioureas occurs in two tautomeric forms. For the parent thiourea, the thione form predominates in aqueous solutions.[2] The thiol form, known as an isothiourea, can be encountered in substituted compounds such as isothiouronium salts.
Synthesis
N,N′-unsubstituted thioureas can be prepared by treating the corresponding cyanamide with hydrogen sulfide or similar sulfide sources.[3] Organic ammonium salts react with potassium thiocyanate as the source of the thiocarbonyl.[4]
Alternatively, N,N′-disubstituted thioureas can be prepared by coupling two amines with thiophosgene:[5]
- 2 R2NH + CSCl2 + 2 (R2N)(R′2N)CS + 2 HCl
Amines also condense with organic thiocyanates to give thioureas:[6]
- R2NH + R′NCS → (R2N)(HR′N)CS
Cyclic thioureas are prepared by transamidation of thiourea with diamines. Ethylene thioureais synthesized by treating ethylenediamine with carbon disulfide.[7] In some cases, thioureas can be prepared by thiation of ureas using phosphorus pentasulfide.

Applications
Precursor to heterocycles
Thioureas are building blocks to pyrimidine derivatives. Thus thioureas condense with β-dicarbonyl compounds.[8] The amino group on the thiourea initially condenses with a carbonyl, followed by cyclization and tautomerization. Desulfurization delivers the pyrimidine. The pharmaceuticals thiobarbituric acid and sulfathiazole are prepared using thiourea.[9] 4-Amino-3-hydrazino-5-mercapto-1,2,4-triazole is prepared by the reaction of thiourea and hydrazine.
Catalysis
Some thioureas are vulcanization accelerators. Thioureas are also used in a research theme called thiourea organocatalysis.[10]
References
- ^ "A Simple Refinement of Density Distributions of Bonding Electrons. IX. Bond Electron Density Distribution in Thiourea, CS(NH2)2, at 123K". Acta Crystallogr. B34 (9): 2789–2794. 1978. doi:10.1107/S0567740878009243.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Allegretti, P.E; Castro, E.A; Furlong, J.J.P (March 2000). "Tautomeric equilibrium of amides and related compounds: theoretical and spectral evidences". Journal of Molecular Structure: THEOCHEM. 499 (1–3): 121–126. doi:10.1016/S0166-1280(99)00294-8.
- ^ Koketsu, Mamoru; Kobayashi, Chikashi; Ishihara, Hideharu (2003). "Synthesis of N-aryl-S-alkylthiocarbamates". Heteroatom Chemistry. 14 (4): 374–378. doi:10.1002/hc.10163.
- ^ Herr, R. J.; Kuhler, L.; Meckler, H.; Opalka, C. J. (2000). "A Convenient Method for the Preparation of Primary and Symmetrical N,N′-Disubstituted Thioureas". Synthesis. 2000 (11): 1569–1574. doi:10.1055/s-2000-7607.
- ^ "Thiourea Based Fluorous Organocatalyst". Top. Curr. Chem. Topics in Current Chemistry. 308: 191–212. 2012. doi:10.1007/128_2011_248. ISBN 978-3-642-25233-4. PMID 21972024.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Discovery and Application of Asymmetric Reaction by Multifunctional Thioureas". Bull Chem Soc Jpn. 81 (7): 785. 2008. doi:10.1246/bcsj.81.785.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ C. F. H. Allen; C. O. Edens; James VanAllan. "Ethylene Thiourea". Organic Syntheses; Collected Volumes, vol. 3, p. 394.
- ^ Foster, H. M., and Snyder, H. R. (1963). "4-Methyl-6-hydroxypyrimidine". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 4, p. 638. - ^ "Thiourea and Thiourea Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. 2002. doi:10.1002/14356007.a26_803. ISBN 3527306730.
{{cite encyclopedia}}: Unknown parameter|authors=ignored (help) - ^ R. Schreiner, Peter (2003). "Metal-free organocatalysis through explicit hydrogen bonding interactions". Chem. Soc. Rev. 32 (5): 289–296. doi:10.1039/b107298f. PMID 14518182.
Further reading
- Patai, S., ed. (1977). The Chemistry of double-bonded functional groups. New York, NY: John Wiley & Sons. pp. 1355–1496. ISBN 0-471-92493-8.