Three-finger protein
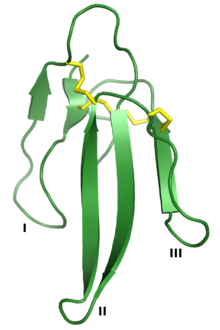
Three-finger proteins or three-finger protein domains (3FP or TFPD) are a protein superfamily consisting of small, roughly 60-80 amino acid residue protein domains with a common tertiary structure: three beta strand loops extended from a hydrophobic core stabilized by disulfide bonds. The family is named for the outstretched "fingers" of the three loops. Members of the family have no enzymatic activity but are capable of forming protein-protein interactions with high specificity and affinity. The founding members of the family, also the best characterized by structure, are the three-finger toxins found in snake venom, which have a variety of pharmacological effects, most typically by disruption of cholinergic signaling. The family is also represented in non-toxic proteins, which have a wide taxonomic distribution; 3FP domains occur in the extracellular domains of some cell-surface receptors as well as in GPI-anchored and secreted globular proteins, usually involved in signaling.[2][3][4][5]
Three-finger toxins
The founding members of the 3FP family are the three-finger toxins (3FTx) often found in snake venom. 3FTx proteins are widely distributed in venomous snake families, but are particularly enriched in the family Elapidae, in which the relative proportion of 3FTx to other venom toxins can reach 95%.[4][6] Many 3FTx proteins are neurotoxins, though the mechanism of toxicity varies significantly even among proteins of relatively high sequence identity; common protein targets include those involved in cholinergic signaling, such as the nicotinic acetylcholine receptors, muscarinic acetylcholine receptors, and acetylcholinesterase. Another large subfamily of 3FTx proteins is the cardiotoxins (also known as cytotoxins or cytolysins); this group is directly cytotoxic most likely due to interactions with phospholipids and possibly other components of the cell membrane.[2]
Ly6/uPAR family

The Ly6/uPAR family broadly describes a gene family containing three-finger protein domains that are not toxic and not venom components; these are often known as LU domains and can be found in the extracellular domains of cell-surface receptors and in either GPI-anchored or secreted globular proteins.[4][8] The family is named for two representative groups of members, the small globular protein lymphocyte antigen 6 (LY6) family and the urokinase plasminogen activator receptor (uPAR).[9] Other receptors with LU domains include members of the transforming growth factor beta receptor (TGF-beta) superfamily, such as the activin type 2 receptor;[10] and bone morphogenetic protein receptor, type IA.[11] Other LU domain proteins are small globular proteins such as CD59 antigen, LYNX1, SLURP1, and SLURP2.[4][12]
Many LU domain containing proteins are involved in cholinergic signaling and bind acetylcholine receptors, notably linking their function to a common mechanism of 3FTx toxicity.[4][8][13] Members of the Ly6/uPAR family are believed to be the evolutionary ancestors of 3FTx toxins.[14] Other LU proteins, such as the CD59 antigen, have well-studied functions in regulation of the immune system.[13]
Gene structure
Snake three-finger toxins and the Ly6/uPAR family members share a common gene structure, typically consisting of two introns and three exons. The sequence of the first exon is generally well conserved compared to the other two.[4] The third exon contains the major differentiating features between the two groups, as this is where the C-terminal GPI-anchor peptide common among the Ly6/uPAR globular proteins is encoded.[4][13]
Evolution and taxonomic distribution
Proteins of the general three-finger fold are widely distributed among metazoans.[4] A 2008 bioinformatics study identified about 45 examples of such proteins, containing up to three three-finger domains, represented in the human genome.[12] A more recent profile of the Ly6/uPAR gene family identified 35 human and at least 61 mouse family members in the organisms' respective genomes.[8]
The three-finger protein family is thought to have expanded through gene duplication in the snake lineage.[14][15] 3FTx toxins are considered restricted to the Caenophidia, the taxon containing all venomous snakes; however at least one homolog has been identified in the Burmese python, a closely related subgroup.[16] Traditionally, 3FTx genes have been thought to have evolved by repeated events of duplication followed by neofunctionalization and recruitment to gene expression patterns restricted to venom glands.[14][15] However, it has been argued that this process should be extremely rare and that subfunctionalization better explains the observed distribution.[17] More recently, non-toxic 3FP proteins have been found to be widely expressed in many different tissues in snakes, prompting the alternative hypothesis that proteins of restricted expression in saliva were selectively recruited for toxic functionality.[16]
References
- ^ Nastopoulos, V.; Kanellopoulos, P. N.; Tsernoglou, D. (1998-09-01). "Structure of dimeric and monomeric erabutoxin a refined at 1.5 A resolution". Acta Crystallographica Section D. 54 (Pt 5): 964–974. ISSN 0907-4449. PMID 9757111.
- ^ a b Kini, RM; Doley, R (November 2010). "Structure, function and evolution of three-finger toxins: mini proteins with multiple targets". Toxicon. 56 (6): 855–67. PMID 20670641.
- ^ Hegde, Raghurama P.; Rajagopalan, Nandhakishore; Doley, Robin; Kini, Manjunatha (2010). "Snake venom three-finger toxins". In Mackessy, Stephen P. (ed.). Handbook of venoms and toxins of reptiles. Boca Raton: CRC Press. pp. 287–302. ISBN 9781420008661.
- ^ a b c d e f g h Kessler, Pascal; Marchot, Pascale; Silva, Marcela; Servent, Denis (2017-03-01). "The three-finger toxin fold: a multifunctional structural scaffold able to modulate cholinergic functions". Journal of Neurochemistry: n/a–n/a. doi:10.1111/jnc.13975. ISSN 1471-4159.
- ^ Utkin, Y; Sunagar, K; Jackson, TNW; Reeks, T; Fry, BG (2015). "Chapter 8: Three-finger toxins". In Fry, Bryan (ed.). Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery. Oxford University Press. pp. 218–227. ISBN 9780199309405.
- ^ Sanz, Libia; Pla, Davinia; Pérez, Alicia; Rodríguez, Yania; Zavaleta, Alfonso; Salas, Maria; Lomonte, Bruno; Calvete, Juan (7 June 2016). "Venomic Analysis of the Poorly Studied Desert Coral Snake, Micrurus tschudii tschudii, Supports the 3FTx/PLA2 Dichotomy across Micrurus Venoms". Toxins. 8 (6): 178. doi:10.3390/toxins8060178.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Leath, Kirstin J.; Johnson, Steven; Roversi, Pietro; Hughes, Timothy R.; Smith, Richard A. G.; Mackenzie, Lloyd; Morgan, B. Paul; Lea, Susan M. (2007-08-01). "High-resolution structures of bacterially expressed soluble human CD59". Acta Crystallographica Section F. 63 (Pt 8): 648–652. doi:10.1107/S1744309107033477. ISSN 1744-3091. PMC 2335151. PMID 17671359.
- ^ a b c Loughner, Chelsea L.; Bruford, Elspeth A.; McAndrews, Monica S.; Delp, Emili E.; Swamynathan, Sudha; Swamynathan, Shivalingappa K. (2016-01-01). "Organization, evolution and functions of the human and mouse Ly6/uPAR family genes". Human Genomics. 10: 10. doi:10.1186/s40246-016-0074-2. ISSN 1479-7364. PMC 4839075. PMID 27098205.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Ploug, Michael; Ellis, Vincent (1994-08-01). "Structure—function relationships in the receptor for urokinase-type plasminogen activator Comparison to other members of the Ly-6 family and snake venom α-neurotoxins". FEBS Letters. 349 (2): 163–168. doi:10.1016/0014-5793(94)00674-1. ISSN 1873-3468.
- ^ Greenwald, Jason; Fischer, Wolfgang H.; Vale, Wylie W.; Choe, Senyon. "Three-finger toxin fold for the extracellular ligand-binding domain of the type II activin receptor serine kinase". Nature Structural Biology. 6 (1): 18–22. doi:10.1038/4887.
- ^ Kirsch, Thomas; Sebald, Walter; Dreyer, Matthias K. "Crystal structure of the BMP-2−BRIA ectodomain complex". Nature Structural Biology. 7 (6): 492–496. doi:10.1038/75903.
- ^ a b Galat, A. (2008-11-01). "The three-fingered protein domain of the human genome". Cellular and Molecular Life Sciences. 65 (21): 3481–3493. doi:10.1007/s00018-008-8473-8. ISSN 1420-682X.
- ^ a b c Tsetlin, Victor I. (2015-02-01). "Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: pharmacological tools and endogenous modulators". Trends in Pharmacological Sciences. 36 (2): 109–123. doi:10.1016/j.tips.2014.11.003. ISSN 1873-3735. PMID 25528970.
- ^ a b c Fry, Bryan G. (2005-03-01). "From genome to "venome": Molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins". Genome Research. 15 (3): 403–420. doi:10.1101/gr.3228405. ISSN 1088-9051. PMC 551567. PMID 15741511.
- ^ a b Fry, Bryan G.; Casewell, Nicholas R.; Wüster, Wolfgang; Vidal, Nicolas; Young, Bruce; Jackson, Timothy N. W. (2012-09-15). "The structural and functional diversification of the Toxicofera reptile venom system". Toxicon. Advancing in Basic and Translational Venomics. 60 (4): 434–448. doi:10.1016/j.toxicon.2012.02.013.
- ^ a b Reyes-Velasco, Jacobo; Card, Daren C.; Andrew, Audra L.; Shaney, Kyle J.; Adams, Richard H.; Schield, Drew R.; Casewell, Nicholas R.; Mackessy, Stephen P.; Castoe, Todd A. (2015-01-01). "Expression of venom gene homologs in diverse python tissues suggests a new model for the evolution of snake venom". Molecular Biology and Evolution. 32 (1): 173–183. doi:10.1093/molbev/msu294. ISSN 1537-1719. PMID 25338510.
- ^ Hargreaves, Adam D.; Swain, Martin T.; Hegarty, Matthew J.; Logan, Darren W.; Mulley, John F. (2014-08-01). "Restriction and Recruitment—Gene Duplication and the Origin and Evolution of Snake Venom Toxins". Genome Biology and Evolution. 6 (8): 2088–2095. doi:10.1093/gbe/evu166. PMC 4231632. PMID 25079342.
