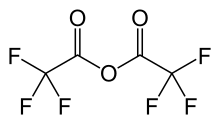
Trifluoroacetic anhydride
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Trifluoroacetic anhydride | |
| Other names
2,2,2-Trifluoroacetic anhydride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.006.349 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4F6O3 | |
| Molar mass | 210.031 g·mol−1 |
| Density | 1.511 g/mL |
| Melting point | −65 °C (−85 °F; 208 K) |
| Boiling point | 40 °C (104 °F; 313 K) |
| Hazards | |
| Safety data sheet (SDS) | Oxford MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trifluoroacetic anhydride (TFAA) is the acid anhydride of trifluoroacetic acid. It is the perfluorinated derivative of acetic anhydride. Like many acid anhydrides, it may be used to introduce the corresponding trifluoroacetyl group. The corresponding acyl chloride, trifluoroacetyl chloride, is a gas, making it inconvenient to work with. Trifluoroacetic anhydride is the recommended desiccant for trifluoroacetic acid.[1]
Preparation
Trifluoroacetic anhydride may be prepared from trifluoroacetic acid by dehydrating with excess α-halogenated acid chlorides. For example, with dichloroacetyl chloride:[2]
- 2 CF3COOH + Cl2CHCOCl → (CF3CO)2O + Cl2CHCOOH + HCl
References
- ^ Chai, Christina Li Lin; Armarego, W. L. F. (2003). Purification of laboratory chemicals (Google Books excerpt). Oxford: Butterworth-Heinemann. p. 376. ISBN 0-7506-7571-3.
- ^ US 4595541
