Trioxidane
| Names | |
|---|---|
| IUPAC name
Trioxidane
| |
| Other names
Dihydrogen trioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 200290 | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H2O3 | |
| Molar mass | 50.013 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
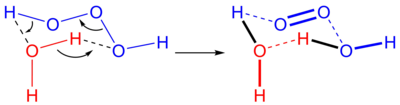
Trioxidane, hydrogen trioxide or dihydrogen trioxide is an unstable molecule with the formula H2O3 or HOOOH. It is one of the hydrogen polyoxides. In aqueous solutions, trioxidane decomposes to form water and singlet oxygen:
The reverse reaction, the addition of singlet oxygen to water, typically does not occur in part due to the scarcity of singlet oxygen. In biological systems, however, ozone is known to be generated from singlet oxygen, and the presumed mechanism is an antibody-catalyzed production of trioxidane from singlet oxygen.[1]
Preparation
Trioxidane can be obtained in small, but detectable, amounts in reactions of ozone and hydrogen peroxide, or by the electrical dissociation of water. Larger quantities have been prepared by the reaction of ozone with organic reducing agents at low temperatures in a variety of organic solvents, and it is also formed during the decomposition of organic hydrotrioxides (ROOOH).[2]
The reaction of ozone with hydrogen peroxide is known as the "Peroxone process". This mixture has been used for some time for treating groundwater contaminated with organic compounds. The reaction produces H2O5.[3]
Structure
Spectroscopic analysis has shown the molecule to have a skewed linear structure H-O-O-O-H, with the O-O bond length being shorter than that in hydrogen peroxide. Various dimeric and trimeric forms also seem to exist. It is slightly more acidic than hydrogen peroxide, dissociating into H+ and OOOH-.[4]
Reactions
Trioxidane readily decomposes into water and singlet oxygen, with a half-life of about 16 minutes in organic solvents at room temperature, but only milliseconds in water. It reacts with organic sulfides to form sulfoxides, but little else is known of its reactivity.
Recent research found that trioxidane is the active ingredient responsible for the antimicrobial properties of the well known ozone / hydrogen peroxide mix. Because these two compounds are present in biological systems as well it is argued that an antibody in the human body can generate trioxidane as a powerful oxidant against invading bacteria.[1][5] The source of the compound in biological systems is the reaction between singlet oxygen and water (which proceeds in either direction, of course, according to concentrations), with the singlet oxygen being produced by immune cells.[2][6]
In 2005, trioxidane was observed experimentally by microwave spectroscopy in a supersonic jet. The molecule exists in a trans conformation with oxygen-oxygen bond lengths of 142.8 picometer compared to 146.4 picometer for hydrogen peroxide. Computational chemistry predicts that more oxygen chain molecules or hydrogen polyoxides exist and that even infinite oxygen chains can exist in a low temperature gas. With this spectroscopic evidence a search for these type of molecules can start in interstellar space.[4]
References
- ^ a b Paul T. Nyffeler, Nicholas A. Boyle, Laxman Eltepu, Chi-Huey Wong, Albert Eschenmoser, Richard A. Lerner, Paul Wentworth Jr. (2004). "Dihydrogen Trioxide (HOOOH) Is Generated during the Thermal Reaction between Hydrogen Peroxide and Ozone". Angewandte Chemie International Edition. 43 (35): 4656–4659. doi:10.1002/anie.200460457. PMID 15317003.
{{cite journal}}: CS1 maint: multiple names: authors list (link)} - ^ a b Božo Plesničar (2005). "Progress in the Chemistry of Dihydrogen Trioxide (HOOOH)". Acta Chim. Slov. 52: 1–12.
- ^ Xin Xu and William A. Goddard III. Peroxonechemistry:Formation of H2O3 and ring-(HO2)(HO3) from O3/H2O2
- ^ a b Kohsuke Suma, Yoshihiro Sumiyoshi, and Yasuki Endo (2005). "The Rotational Spectrum and Structure of HOOOH". J. Am. Chem. Soc. 127 (43): 14998–14999. doi:10.1021/ja0556530. PMID 16248618.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ A Time-Honored Chemical Reaction Generates an Unexpected Product, News & Views, September 13, 2004
- ^ Roald Hoffmann (2004). "The Story of O". American Scientist.