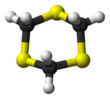
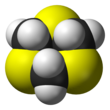
1,3,5-Trithiane

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3,5-Trithiane | |||
| Other names
Thioformaldehyde trimer, Trimethylentrisulfide, Trimethylene trisulfide, Trithioformaldehyde, 1,3,5-Trithiacyclohexane, sym-Trithiane, Thioform, s-Trithiane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.482 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H6S3 | |||
| Molar mass | 138.27 | ||
| Appearance | Colourless solid | ||
| Density | 1.6374 g/cm3[1] | ||
| Melting point | 215 to 220 °C (419 to 428 °F; 488 to 493 K) | ||
| Slightly soluble | |||
| Solubility | Benzene | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Toxic (T) | ||
| GHS labelling: | |||

| |||
| Warning | |||
| H319 | |||
| P264, P280, P305+P351+P338, P313 | |||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
1,3,5-Trithiane is the chemical compound with the formula (CH2S)3. This heterocycle is the cyclic trimer of the otherwise unstable species thioformaldehyde. It consists of a six-membered ring with alternating methylene bridges and thioether groups. It is prepared by treatment of formaldehyde with hydrogen sulfide.[2]
Trithiane is a building block molecule in organic synthesis, being a masked source of formaldehyde. In one application, it is deprotonated with organolithium reagents to give the lithium derivative, which can be alkylated.[3]
- (CH2S)3 + RLi → (CH2S)2(CHLiS) + RH
- (CH2S)2(CHLiS) + R′Br → (CH2S)2(CHR′S) + LiBr
- (CH2S)2(CHR′S) + H2O → R′CHO + ....
Trithiane is the dithioacetal of formaldehyde. Other dithioacetals undergo similar reactions to the above.
It is also a precursor to other organosulfur reagents. For example, chlorination in the presence of water affords the chloromethyl sulfonyl chloride:[4]
- (CH2S)3 + 9 Cl2 + 6 H2O → 3 ClCH2SO2Cl + 12 HCl

Trithianes[edit]
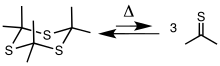
Trithiane is the parent of a class of heterocycles called trithianes, that formally result from substitution of various monovalent groups for one or more of the hydrogen atoms. The species often arise from thiation of ketones and aldehydes. The incipient thioketones and thioaldehydes undergo trimerization. One example is 2,2,4,4,6,6-hexamethyl-1,3,5-trithiane, or trithioacetone, the trimer of thioacetone (propane-2-thione). Alternatively 1,3,5-trithiane can be deprotonated and alkylated to afford (SCH2)n(SCHR)3-n.[5]
The conformation of trithianes has been well investigated.[6]
References[edit]
- ^ David R. Lide, ed. Handbook of Chemistry and Physics, 85th Edition, Internet Version 2005. CRC Press, 2005.
- ^ Bost, R. W.; Constable, E. W. "sym-Trithiane" Organic Syntheses, Collected Volume 2, p.610 (1943). http://orgsyn.org/Content/pdfs/procedures/CV2P0610.pdf
- ^ Seebach, D.; Beck, A. K. "Aldehydes From sym-Ttrithiane: n-Pentadecanal" Organic Syntheses, Collected Volume 6, p.869 (1988). http://orgsyn.org/Content/pdfs/procedures/CV6P0869.pdf
- ^ Paquette, L. A.; Wittenbrook, L. S. "2-Chlorothiirane 1,1-Dioxide" Organic Syntheses, Collected Volume 5, p.231 (1973). http://orgsyn.org/Content/pdfs/procedures/CV5P0231.pdf
- ^ Edema, Jilles J. H.; Hoogenraad, Marcel; Schoonbeek, Franck S.; Kellogg, Richard M.; Kooijman, Huub; Spek, Anthony L. (2010). "Alkylation of the SCS linkage. Towards lipophilic mono- and ditopic heavy-metal receptors containing trithiane building blocks. Molecular structure of cis-2,4,6-tribenzyl-1,3,5-trithiane". Recueil des Travaux Chimiques des Pays-Bas. 112 (6): 370–375. doi:10.1002/recl.19931120611.
- ^ Breslow, David S.; Skolnik, Herman (2009). "C3S3 Ring Systems". Multi-Sulfur and Sulfur and Oxygen Five- and Six-Membered Heterocycles, Part 2. Vol. 21. John Wiley & Sons. p. 689. ISBN 978-0-470-18833-0.