Tropylium cation
In organic chemistry, the tropylium ion is an aromatic species with a formula of [C7H7]+.[1] Its name derives from the molecule tropane (itself named for the molecule atropine).
It is a heptagonal, planar, cyclic ion; as well, it has 6 π-electrons (4n+ 2, where n=1), which fulfills Hückel's rule of aromaticity. It can coordinate as a ligand to metal atoms.
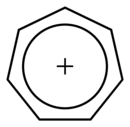 |
 |
 |
The structure shown is a composite of seven resonance contributors in which each carbon carries part of the positive charge.
Mass spectrometry
The tropylium ion is frequently encountered in mass spectrometry in the form of a signal at m/z = 91 and is used in mass spectrum analysis. This fragment is often found for aromatic compounds containing a benzyl unit. Upon ionization, the benzyl fragment is cleaved off as a cation (PhCH2+), which rearranges to the highly stable tropylium cation (C7H7+).

Note parent peak corresponding to molecular mass M = 92 (C7H8+) and
highest peak at M-1 = 91 (C7H7+, quasi-stable tropylium cation).
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "molecule". doi:10.1351/goldbook.M04002
