Aromatic sulfonation
In organic chemistry, aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid (−SO2OH) functional group in an electrophilic aromatic substitution.[1] Aryl sulfonic acids are used as detergents, dye, and drugs.
Stoichiometry and mechanism
[edit]
Typical conditions involve heating the aromatic compound with sulfuric acid:[2]
- C6H6 + H2SO4 → C6H5SO3H + H2O
Sulfur trioxide or its protonated derivative is the actual electrophile in this electrophilic aromatic substitution.
To drive the equilibrium, dehydrating agents such as thionyl chloride can be added.[citation needed]
- C6H6 + H2SO4 + SOCl2 → C6H5SO3H + SO2 + 2 HCl
Historically, mercurous sulfate has been used to catalyze the reaction.[3]
Chlorosulfuric acid is also an effective agent:
- C6H6 + HSO3Cl → C6H5SO3H + HCl
In contrast to aromatic nitration and most other electrophilic aromatic substitutions this reaction is reversible. Sulfonation takes place in concentrated acidic conditions and desulfonation is the mode of action in a dilute hot aqueous acid. The reaction is very useful in protecting the aromatic system because of this reversibility. Due to their electron withdrawing effects, sulfonate protecting groups can be used to prevent electrophilic aromatic substitution. They can also be installed as directing groups to affect the position where a substitution may take place.[4]
Specialized sulfonation methods
[edit]Many method have been developed for introducing sulfonate groups aside from direction sulfonation.
A classic named reaction is the Piria reaction (Raffaele Piria, 1851) in which nitrobenzene is reacted with a metal bisulfite forming an aminosulfonic acid as a result of combined nitro group reduction and sulfonation.[5][6]
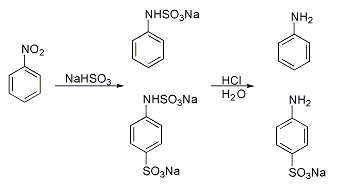
In the Tyrer sulfonation process (1917),[7] at some time of technological importance, benzene vapor is led through a vessel containing 90% sulfuric acid the temperature of which is increased from 100 to 180°C. Water and benzene are continuously removed and the benzene fed back to the vessel. In this way an 80% yield is obtained.

Applications
[edit]
Aromatic sulfonic acids are intermediates in the preparation of dyes and many pharmaceuticals. Sulfonation of anilines lead to a large group of sulfa drugs.
Sulfonation of polystyrene is used to make sodium polystyrene sulfonate, a common ion exchange resin for water softening.
See also
[edit]References
[edit]- ^ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 3rd edition, New York: Wiley, ISBN 9780471854722, OCLC 642506595.
- ^ Otto Lindner, Lars Rodefeld "Benzenesulfonic Acids and Their Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim.doi:10.1002/14356007.a03_507
- ^ Sabatier, Paul (1922). Catalysis in Organic Chemistry. Translated by Reid, E. Emmet. New York, NY: Van Nostrand. p. 2.
- ^ T.W. Graham Solomons: Organic Chemistry, 11th Edition, Wiley, Hoboken, NJ, 2013, p. 676, ISBN 978-1-118-13357-6.
- ^ Piria, Raffaele (1851). "Über einige Produkte der Einwirkung des schwefligsäuren Ammoniaks auf Nitronaphtalin". Annalen der Chemie und Pharmacie. 78: 31–68. doi:10.1002/jlac.18510780103. ISSN 0075-4617.
- ^ THE PIRIA REACTION. I. THE OVER-ALL REACTION W. H. Hunter, Murray M. Sprung J. Am. Chem. Soc., 1931, 53 (4), pp 1432–1443 doi:10.1021/ja01355a037.
- ^ U.S. patent 1,210,725
- ^ Siegfried Hauptmann: Organische Chemie, 2nd Edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 511, ISBN 3-342-00280-8.

