Uronic acid
- For hexuronic acid, i.e. the older name of Vitamin C, see Ascorbic acid.

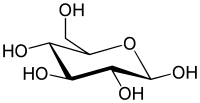

Uronic acids (/jʊˈrɒn[invalid input: 'ɨ']k/) are a class of sugar acids with both carbonyl and carboxylic acid functional groups. They are sugars in which the terminal carbon's hydroxyl group has been oxidized to a carboxylic acid. Oxidation of the terminal aldehyde instead yields an aldonic acid, while oxidation of both the terminal hydroxyl group and the aldehyde yields an aldaric acid. The names of uronic acids are generally based on their parent sugars, however some of the most common do not have direct parents, and are formed by epimerization (e.g., iduronic acid is an epimer of glucuronic acid). Uronic acids that have six carbons are called hexuronic acids.
Examples
Some of these compounds have important biochemical functions; for example, many wastes in the human body are excreted in the urine as their glucuronate salts, and iduronic acid is a component of some structural complexes such as proteoglycans.
External links
- Uronic+Acids at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Description at zvon.org
- Synthesis at chembio.uoguelph.ca (halfway down page.)
