User:Bruce L. Burton/sandbox
The portion below the following line is intended to replace only the introductory paragraph and the first section entitled "Epoxy". Some minor edits have already been made to other portions of the article. -- Bruce Burton
Epoxy (/ə-POCK-see/), a shortened term for "epoxy resin", can refer to either the resinous starting component(s) of an epoxy resin "formulation", or to the polymerized product. The formulation is recipe of the mixture used to create the final material. It may contain not only the reactive components of epoxy resins and cross-linking agents (also called hardeners) but also non-reactive liquids and solids added for improvements in processing, performance, and/or cost reduction. The polymerized product is typically a thermosetting plastic (a network polymer) and results from reaction of starting mixture. This final material may still be referred to as an epoxy, even though the polymerized, plastic material typically retains very few of the epoxide (a.k.a. oxirane) functional groups of the starting resin. This article covers both the starting materials, consisting of a resin, a hardener (also called the crosslinker, crosslinking agent, co-reactant, or catalyst) and the polymerized materials that result from the reaction of the starting materials with one another. Note that the polymerized, plastic material typically retains very few of the epoxide (a.k.a. oxirane) functional groups of the starting resin but is still referred to as epoxy resin.


Epoxy resin systems are widely known for their useful combinations of strength, modulus, solvent resistance, corrosion resistance, and adhesion. As for many plastics, the choice to use an epoxy is often based on a combination of its processing behavior, cost-effectiveness, and the mechanical and thermal properties of the cured (cross-linked) polymer. Epoxy resins are most commonly used in coating formulations, for electrical and electronic applications (see further below), and as the matrix material for composite materials such as fiber-wound pipes, storage tanks, and air-foils.
The history of epoxy resin technology is summarized in a review article[1]. (As a note of caution when using the article by Pham and Marks, the numbering is wrong in most of the reference section.)"
BLB below------------
The history of epoxy resin technology is summarized in a review article[1]. (As a note of caution when using the article by Pham and Marks, the numbering is wrong in most of the reference section.)
Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. Epoxy resins may be reacted (cross-linked) either with themselves through catalytic homopolymerisation, or with a wide range of co-reactants including polyfunctional amines, acids (and acid anhydrides), phenols (polyphenols and phenolic resins), thiols (usually called mercaptans), and polyols. These co-reactants are often referred to as hardeners or curatives, and the cross-linking reaction is commonly referred to as curing. Reaction of polyepoxides with themselves or with polyfunctional hardeners forms a thermosetting polymer, often with favorable combinations of mechanical properties and the desired level of thermal and chemical resistance. Epoxy formulations have a wide range of applications, including coatings (for both metal and wood), use in electronics/electrical components/LEDs, high voltage electrical insulators, transformer coil windings, semi-conductor encapsulants, paint brush manufacturing, fiber-reinforced plastic materials and structural adhesives.
Epoxy resin[edit]
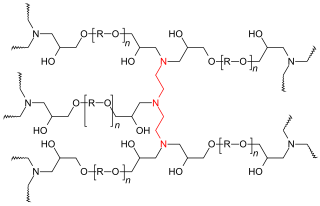
Epoxy resins are low molecular weight pre-polymers or higher molecular weight polymers which normally contain at least two epoxide groups. The epoxide group is also sometimes referred to as a glycidyl or oxirane group.
A wide range of epoxy resins are produced industrially. The raw materials for epoxy resin production are today largely petroleum derived, although some plant derived sources are commercially available (e.g. plant derived glycerol used to make epichlorohydrin).
The most widely available epoxy resins are polymeric or semi-polymeric materials or an Oligomer, and as such are usually not pure substances, since mixtures of variable chain lengths result during the polymerisation reaction used to produce them. High purity grades can be produced for certain applications, e.g. using distillation or crystallization processes for purification. One downside of high purity liquid grades is their tendency to form crystalline solids (due to their highly regular structure) which require melting and mixing to enable processing.
An important criterion for epoxy resins is the epoxide group content. This is correctly expressed as the specific amount of substance of epoxide groups in the material B under consideration, calculated as the ratio of the amount of substance of epoxide groups in this material B, n(EP), divided by the mass m(B) of the material B under consideration, in this case, the mass of the resin. The SI unit for this quantity is "mol/kg", or multiples thereof. Deprecated quantities which are still in use are the so-called "epoxide number" which is (not a number and should therefore not be referred to as such, but) the ratio of the amount of substance of epoxide groups, n(EP), and the mass m(B) of the material B, with the SI unit "mol/kg", or the so-called "epoxide equivalent weight", which is the ratio of the mass of a sample B of the resin and the amount of substance of epoxide groups present in that sample B, with the SI unit "kg/mol". The so-called "epoxide equivalent weight" is simply the inverse of the so-called "epoxide number", though in common practice the EEW is adjusted to be expressed as grams per epoxide equivalent.
The specific amount of substance of epoxide groups is used to calculate the mass of co-reactant (hardener) to use when curing epoxy resins. Epoxies are typically cured with stoichiometric or near-stoichiometric quantities of curative to achieve maximum physical properties. Achieving the maximum glass transition temperature is of particular importance for many applications.
Similar to other classes of thermoset polymer materials, blending different types and grades of epoxy resins and hardeners, as well as the incorporation of additives such as plasticizers, fillers, or flame retardants, is commonly used to achieve desired processing, performance, and cost objectives. The use of resin blending, hardener blending, additives. and fillers is often referred to as formulating.
- ^ Pham, Ha Q.; Marks, Maurice J. (2005). Ullman's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH Verlag GmbH & Co.
