User:Carissa92/sandbox/Final(Neuroscience-of-sex-differences)
Hormones[edit]
Gonadal hormones, or sex hormones, include androgens (such as testosterone) and estrogens (such as estradiol) which are synthesized primarily in the testes and ovaries, respectively. Sex hormone production is regulated by the gonadotropic hormones luteinizing hormone (LH) and follicle-stimulating hormone (FSH), whose release from the anterior pituitary is stimulated by gonadotropin-releasing hormone (GnRH) from the hypothalamus.[1] These steroid hormones have several effects on brain development as well as on maintenance of homeostasis throughout adulthood.[2] For instance, estrogen receptors have been found in the hypothalamus, pituitary gland, hippocampus, and frontal cortex, indicating the estrogen plays a role in brain development. Gonadal hormone receptors have also been found in the basal fore-brain nuclei.[3]
Estrogen[edit]
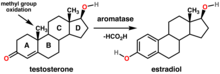

Estrogens are a class of hormones that are synthesized from androgens, such as testosterone, in both males in females. About half of the testosterone made in the ovaries is converted via the enzyme aromatase to estradiol, the primary estrogen in humans.[1]
During development, estrogen may promote either feminizing and masculinizing effects on the human brain; high levels of estrogen induce male neural traits to develop while moderate levels induce female traits. In females, masculinizing effects are resisted because of the presence of α-fetoprotein (AFP), a carrier protein proposed to transport estrogen into brain cells, allowing the female brain to properly develop. Prenatally, AFP blocks estrogen, whereas postnatally AFP decreases to ineffective levels. Therefore, it is probable that estrogen exhibits its effects on female brain development postnatally.[4]
Estrogen may influence cognitive function by modulating learning and memory in a dose-sensitive manner. Ovariectomized female rats administered doses of estrogen and progesterone to mimic peak levels during the estrous cycle initially showed impaired learning on a swim task as compared to the untreated group, perhaps as a result of the interaction between estrogen and high levels of stress. During subsequent trials, however, estrogen-treated rats performed better, indicating the effects of estrogen may actually enhance working memory despite promoting a less effective learning approach initially.[5]
Ovariectomies, surgeries inducing menopause, or natural menopause cause fluctuating and decreased estrogen levels in women. This in turn can "attenuate the effects" of endogenous opioid peptides. Opioid peptides are known to play a role in emotion and motivation. β-endorphin (β-EP), an endogenous opioid peptide, content has been found to decrease (in varying amounts/brain region), post ovariectomy, in female rats within the hypothalamus, hippocampus, and pituitary gland. Such a change in β-EP levels could be the cause of mood swings, behavioral disturbances, and hot flashes in post-menopausal women.[3]
Progesterone[edit]
The steroid hormone progesterone is primarily synthesized in the ovaries, but is also produced by the adrenal glands and, to a lesser extent, the central nervous system in both males and females. Progesterone levels in males are typically around 1-3 nM, which is similar to levels in women during the follicular phase of the menstrual cycle or during menopause, but can rise significantly in response to physical or psychological stressors. In women, progesterone and its metabolite allopregnanolone are important in the menstrual cycle, with levels reaching from 20-40 nM during the luteal phase, and in maintaining pregnancy, with a peak of around 200-400 nM during the third trimester.[6]
In female rats, progesterone and allopregnanolone stimulate the sexual behavior lordosis, the posture adopted in response to mounting by a male rat. Progesterone may exert anxiolytic, antidepressant, and neuroprotective effects, such as through facilitating remyelination following peripheral nerve damage, in both men and women.[6]

Testosterone[edit]
The gonadal hormone testosterone is an androgenic, or masculinizing, hormone that is synthesized in both the male testes and female ovaries,[7] at a rate of about 14000 μg/day and 600 μg/day, respectively.[1] Testosterone exerts organizational effects on the developing brain, many of which are mediated through its conversion to estrogen by the enzyme aromatase.[1] The prenatal female brain is protected from the masculinizing effects of estrogen through the presence of α-fetoprotein (AFP).[4]
Testosterone has been shown to influence proaptotic proteins to induce neuronal cell death in certain brain regions while aiding in the construction of others.[2] Development of the larger male sexually dimorphic preoptic area is contingent on the presence of androgens during fetal development in rats, while the development of a denser anteroventral periventricular nucleus in the hypothalamus of female rats only occurs in the absence of androgens.[7] Female rats introduced to elevated testosterone levels perinatally will exhibit typical male sexual behaviors, such as mounting, as adults.[2][7]
Prolactin[edit]
Prolactin, the peptide hormone known for its role in lactation and other maternal behavior, is synthesized primarily in the pituitary. However prolactin expression is also found in the hypothalamus and cerebellum in humans and has been observed throughout various cortical and subcortical brain regions in other animal models, such as rats and sheep, where it has been implicated in cognition, neurogenesis, and neuroprotective functions. The elevated levels of prolactin observed during pregnancy and nursing appear to have an anxiolytic effect in female rats.[8]
Although less is known about the role of prolactin in males, it seems to play a role in aspects of male sexual function and the promotion of certain paternal behaviors. Low levels of prolactin are correlated with both sexual dysfunction and increased anxiety in human males.[9]
Vasopressin and Oxytocin[edit]
Oxytocin is positively correlated with maternal behaviors, social recognition, social contact, sexual behavior and pair bonding. Oxytocin appears at higher levels in women than in men, while vasopressin is present in higher levels in males and mediates sexual behavior, aggression, and other social functions.[10]
References[edit]
- ^ a b c d Molina, Patricia E. (2018). Endocrine physiology (5th ed.). New York: McGraw-Hill Education. ISBN 9781260019360. OCLC 1026417940.
- ^ a b c Simerly, Richard B. (2005). "Wired on hormones: endocrine regulation of hypothalamic development". Current Opinion in Neurobiology. 15 (1): 81–85. doi:10.1016/j.conb.2005.01.013. ISSN 0959-4388. PMID 15721748.
- ^ a b Genazzani, Andrea Riccardo; Pluchino, Nicola; Luisi, Stefano; Luisi, Michele (2007). "Estrogen, cognition and female ageing". Human Reproduction Update. 13 (2): 175–187. doi:10.1093/humupd/dml042. ISSN 1355-4786. PMID 17135285.
- ^ a b Bakker, Julie; Baum, Michael J. (2008). "Role for estradiol in female-typical brain and behavioral sexual differentiation". Frontiers in Neuroendocrinology. 29 (1): 1–16. doi:10.1016/j.yfrne.2007.06.001. ISSN 1095-6808. PMC 2373265. PMID 17720235.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Korol, Donna L. (2004). "Role of estrogen in balancing contributions from multiple memory systems". Neurobiology of Learning and Memory. 82 (3): 309–323. doi:10.1016/j.nlm.2004.07.006. ISSN 1074-7427. PMID 15464412.
- ^ a b Schumacher, M.; Mattern, C.; Ghoumari, A.; Oudinet, J.P.; Liere, P.; Labombarda, F.; Sitruk-Ware, R.; De Nicola, A.F.; Guennoun, R. (2014). "Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors". Progress in Neurobiology. 113: 6–39. doi:10.1016/j.pneurobio.2013.09.004.
- ^ a b c Hadley, Mac E.; Levine, Jon E. (2007). Endocrinology. Levine, Jon E. (6th ed.). Upper Saddle River, NJ: Pearson Prentice Hall. ISBN 0131876066. OCLC 70929277.
- ^ Cabrera-Reyes, Erika Alejandra; Limón-Morales, Ofelia; Rivero-Segura, Nadia Alejandra; Camacho-Arroyo, Ignacio; Cerbón, Marco (2017-08-01). "Prolactin function and putative expression in the brain". Endocrine. 57 (2): 199–213. doi:10.1007/s12020-017-1346-x. ISSN 1559-0100.
- ^ Rastrelli, Giulia; Corona, Giovanni; Maggi, Mario (2015-09-01). "The role of prolactin in andrology: what is new?". Reviews in Endocrine and Metabolic Disorders. 16 (3): 233–248. doi:10.1007/s11154-015-9322-3. ISSN 1573-2606.
- ^ Carter, C. Sue (2007). "Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders?". Behavioural Brain Research. 176 (1): 170–186. doi:10.1016/j.bbr.2006.08.025. ISSN 0166-4328. PMID 17000015.
