User:JasonLin366123/chem275
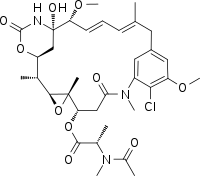
| |
| Names | |
|---|---|
| Other names
Maitansine
Maytansin MAYTANSINE Maysanine MLS002703019 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C34H46ClN3O10 | |
| Molar mass | 692.20 g/mol |
| Appearance | white to beige; powder |
| DMSO: 2 mg/mL, clear | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
General information
[edit]Maitansine (INN), or maytansine (USAN), is a cytotoxic agent. It inhibits the assembly of microtubules by binding to tubulin at the rhizoxin binding site.[1][2]It is a macrolide of the ansamycin type and can be isolated from plants of the genus Maytenus.[1]
Maytansine is a potent microtubule-targeting agent featuring a 19-member ansa macrolide structure attached to a chlorinated benzene ring.[2][3] Initially isolated from the Ethiopian shrub Maytenus serrata and later from Maytenus ovatus, it binds to tubulin at the rhizoxin binding site, disrupting microtubule assembly and inducing mitotic arrest.[4][5][6] Although highly effective at sub-nanomolar concentrations in inhibiting microtubule assembly, maytansine's lack of tumor specificity and significant side effects have limited its clinical use as a standalone drug.[2][7] However, its derivatives, particularly when used as part of antibody-drug conjugates (ADCs), show promising results in targeted cancer therapy. Maytansine and other natural products like it are particularly valuable because they offer diverse structural classes and mechanisms of action within the same functional group.[7]
Maytansinoids
[edit]Derivatives of maitansine are known as maytansinoids.[8][9] Some are being investigated as the cytotoxic component of antibody-drug conjugates for cancer treatment,[10] and the antibody-drug conjugate trastuzumab emtansine is an approved drug for the treatment of certain kinds of breast cancer in the EU and in the US.[11][12]
Examples of maytansinoids are:
- Ansamitocin[8]
- Mertansine / emtansine (DM1)[3]
- Ravtansine / soravtansine (DM4)[3]

History, discovery, and development
[edit]Maytansine was first discovered by Kupchan and coworkers in 1972 from the plant Maytenus ovatus, and later shown to be produced by microbial endophytes.[7][13] Recognized for its potent antileukemic activity and unique chemical structure. Later studies demonstrated that the potent cytotoxicity and in vivo antitumor activity were the result of potent binding to tubulin at the Vinca alkaloid site.[7][14][15] Due to the powerful antitumor activity coupled with the relative scarcity of the compound, maytansine led to significant synthetic and clinical research efforts throughout the 1970s and 1980s. These studies initially revealed its potential in treating various cancers, but clinical trials were largely unsuccessful due to its toxicity and inefficacy at safe dosages.[7] A resurgence in interest came with the development of ADCs, which utilize maytansine's cytotoxicity in a targeted manner, improving safety and efficacy.[3][7]
Toxicity and adverse effects
[edit]Maytansine exhibits severe toxicity, which has been a major barrier to its use as a standard cancer therapy.[13] The primary issues include dose-limiting toxicities, with adverse effects so significant that they led to the cessation of initial clinical trials. Its systemic toxicity, particularly neurotoxicity, arises from its mechanism of action that disrupts microtubules, a critical component of cell and axonal structure.[7]
In preclinical studies, maytansine was administered subcutaneously to 5-week-old male F344 rats at a LD50 (14 days) of 0.48 mg/kg.[4]Toxicological evaluation showed that maytansine has a potent effect on rapidly dividing cells and can cause severe lesions in tissues with high rates of cell division. Its effect on cell division was highlighted by histologic findings of mitotic divisions in many tissues within hours of dosing. Subsequent necrotic lesions lead to atrophic changes in the gastrointestinal tract, thymus, spleen, bone marrow, and testes.[3][4] Maytansine also causes parenchymal and cerebral hemorrhagic lesions with mononuclear infiltration around meningeal vessels and pigmentolysis and vacuolization of dorsal root ganglion cells. These cellular changes were accompanied by clinical manifestations of ataxia, suggesting that the drug has widespread systemic effects. In addition, skin ulcers developed at the site of administration.[4]
Pharmaceutical Effects
[edit]Recent developments in ADC technology have leveraged the potent cytotoxicity of maytansine analogs such as S-methyl DM1 and S-methyl DM4, primary cellular metabolites from thiol-containing maytansinoids DM1 and DM4.[7][16] These derivatives, though less potent in polymerization inhibition than maytansine, have been shown to suppress microtubule dynamic instability more effectively, acting as potent microtubule poisons.[3][7] The recent Phase II clinical trial with trastuzumab-DM1, a maytansinoid conjugate, demonstrated an overall response rate of 39% in patients with metastatic breast cancer, showing significant promise.[3] In cells, these conjugates undergo lysosomal degradation; the resulting metabolites continue to impact tubulin dynamics, highlighting their potential as the active intracellular components of ADCs. This advancement highlights a significant step forward in utilizing maytansine's properties in a manner that targets cancer cells more effectively while minimizing impact on normal tissues.[3]
Synthesis and Chemistry
[edit]The synthesis of thiol-containing maytansinoids DM1 and DM4 involves specific chemical reactions under controlled conditions. DM1 [N2'-deacetyl-N2'-(3-mercapto-1-oxopropyl)-maytansine] and DM4 [N2'-deacetyl-N2'-(4-mercapto-4-methyl-1-oxopentyl)-maytansine] are first prepared following established protocols. The synthesis process for their thiomethyl derivatives involves reacting each compound overnight with an excess of methyl iodide and N,N-diisopropylethylamine in a N,N-dimethylformamide (DMF) solution. This step converts DM1 and DM4 into their respective thiomethyl derivatives: S-methyl DM1 [N2'-deacetyl-N2'-(3-thiomethyl-1-oxopropyl)-maytansine] and S-methyl DM4 [N2'-deacetyl-N2'-(4-thiomethyl-4-methyl-1-oxopentyl)-maytansine].[3]
See also
[edit]- ImmunoGen, developer of maytansinoid based drugs
References
[edit]- ^ a b National Cancer Institute: Definition of Maytansine
- ^ a b c PubChem. "Maitansine". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-05-06.
- ^ a b c d e f g h i Lopus, Manu; Oroudjev, Emin; Wilson, Leslie; Wilhelm, Sharon; Widdison, Wayne; Chari, Ravi; Jordan, Mary Ann (2010-10-01). "Maytansine and Cellular Metabolites of Antibody-Maytansinoid Conjugates Strongly Suppress Microtubule Dynamics by Binding to Microtubules". Molecular Cancer Therapeutics. 9 (10): 2689–2699. doi:10.1158/1535-7163.MCT-10-0644. ISSN 1535-7163. PMC 2954514. PMID 20937594.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b c d Mugera, G. M.; Ward, J. M. (1977-10). "Acute toxicity of maytansine in F344 rats". Cancer Treatment Reports. 61 (7): 1333–1338. ISSN 0361-5960. PMID 563288.
{{cite journal}}: Check date values in:|date=(help) - ^ Pyle, S.J.; Reuhl, K.R. (2010), "Cytoskeletal Elements in Neurotoxicity*", Comprehensive Toxicology, Elsevier, pp. 71–87, doi:10.1016/b978-0-08-046884-6.01306-3, ISBN 978-0-08-046884-6, retrieved 2024-05-06
- ^ Mandelbaum-Shavit, Frederika; Wolpert-DeFilippes, Mary K.; Johns, David G. (1976-09). "Binding of maytansine to rat brain tubulin". Biochemical and Biophysical Research Communications. 72 (1): 47–54. doi:10.1016/0006-291x(76)90958-x. ISSN 0006-291X.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d e f g h i Natural Products and Cancer Drug Discovery. doi:10.1007/978-1-4614-4654-5.
- ^ a b Yu, T.-W.; Bai, L; Clade, D; Hoffmann, D; Toelzer, S; Trinh, KQ; Xu, J; Moss, SJ; Leistner, E (2002). "The biosynthetic gene cluster of the maytansinoid antitumor agent ansamitocin from Actinosynnemapretiosum". Proceedings of the National Academy of Sciences. 99 (12): 7968–7973. Bibcode:2002PNAS...99.7968Y. doi:10.1073/pnas.092697199. PMC 123004. PMID 12060743.
- ^ Lopus, M; Oroudjev, E; Wilson, L; Wilhelm, S; Widdison, W; Chari, R; Jordan, MA (2010). "Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules". Mol Cancer Ther. 9 (10): 2689–99. doi:10.1158/1535-7163.MCT-10-0644. PMC 2954514. PMID 20937594.
- ^ Chari, RV; Martell, BA; Gross, JL; et al. (January 1992). "Immunoconjugates containing novel maytansinoids: promising anticancer drugs" (PDF). Cancer Res. 52 (1): 127–31. PMID 1727373.
- ^ "Kadcyla EPAR". European Medicines Agency (EMA). 17 September 2018.
- ^ "Drug Approval Package: ado-trastuzumab emtansine". U.S. Food and Drug Administration (FDA). 22 February 2013. Archived from the original on 4 December 2019. Retrieved 3 December 2019.
- ^ a b Atanasov, Atanas G.; Zotchev, Sergey B.; Dirsch, Verena M.; Supuran, Claudiu T. (2021-03). "Natural products in drug discovery: advances and opportunities". Nature Reviews Drug Discovery. 20 (3): 200–216. doi:10.1038/s41573-020-00114-z. ISSN 1474-1784. PMC 7841765. PMID 33510482.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Bhattacharyya, B.; Wolff, J. (1977-03-15). "Maytansine binding to the vinblastine sites of tubulin". FEBS Letters. 75 (1–2): 159–162. doi:10.1016/0014-5793(77)80075-6. ISSN 0014-5793.
- ^ Huang, Abbott B.; Lin, Chii M.; Hamel, Ernest (1985-05). "Maytansine inhibits nucleotide binding at the exchangeable site of tubulin". Biochemical and Biophysical Research Communications. 128 (3): 1239–1246. doi:10.1016/0006-291x(85)91073-3. ISSN 0006-291X.
{{cite journal}}: Check date values in:|date=(help) - ^ Issell, Brian F.; Crooke, Stanley T. (1978-12). "Maytansine". Cancer Treatment Reviews. 5 (4): 199–207. doi:10.1016/s0305-7372(78)80014-0. ISSN 0305-7372.
{{cite journal}}: Check date values in:|date=(help)
