User:Kevinxchan/sandbox
 | This is a user sandbox of Kevinxchan. You can use it for testing or practicing edits. This is not the sandbox where you should draft your assigned article for a dashboard.wikiedu.org course. To find the right sandbox for your assignment, visit your Dashboard course page and follow the Sandbox Draft link for your assigned article in the My Articles section. |
Original- "Exoelectrogen"
Extracellular electron transport mechanisms
Reduced oxidoreductase enzymes at the extracellular membrane have been shown to use the following methods in transferring their electrons to the exogenous final acceptor: direct contact, shuttling via excreted mediators, through a conductive biofilm, and through conductive pili (Figure 2); additionally, the possibility exists that these methods are not mutually exclusive.[1]
Direct reduction of an exogenous acceptor is done through direct contact between it and the final oxidoreductase. In addition, the presence of electron shuttling molecules dramatically increases the transfer rate. In S. oneidensis MR-1, flavins are secreted that will oxidize MtrC oxidoreductase and can increase the rate of transfer by up to 80%.[2]
Edits- "Exoelectrogen"
Extracellular electron transport mechanisms
Reduced oxidoreductase enzymes at the extracellular membrane have been shown to use the following methods in transferring their electrons to the exogenous final acceptor: direct contact, shuttling via excreted mediators, iron chelating agents[3], through a conductive biofilm, and through conductive pili (Figure 2); additionally, the possibility exists that these methods are not mutually exclusive.[1]
Iron chelation is a process by which insoluble ferric oxide compounds are solubilized in aqueous solutions. As bioavailability of iron is scarce, many microbes secrete iron chelating compounds to solubilize, uptake, and sequester iron for various cellular processes. Certain microorganisms have been shown to be capable of using such compounds for electron transport by solubilizing iron extracellularly[4]. The components used in each pathway varies between species are phylogenetically diverse[5], thus some chelating agents may reduce iron outside the cell acting as electron shuttles, while others may deliver iron to the cell for membrane bound reduction[4]. The source of iron chelating agents can be heterogeneous, ranging from organic and inorganic molecules, with molecules synthesized inside or outside the cell[4].
Direct reduction of an exogenous acceptor is done through direct contact between it and the final oxidoreductase. In addition, the presence of electron shuttling molecules dramatically increases the transfer rate. In the model organism S. oneidensis MR-1, transport is characterized by the proteins CymA, STC, FccA, MtrA, MtrB, MtrC, and OmcA. Together, these form an electron transport chain starting from the cytoplasmic membrane to outer surface of the cell, where MtrC and OmcA can interact and transport electrons to the mineral compound. Flavins are secreted which are thought to bind to OmcA and MtrC (the two shuttling molecules) which increases the reduction potential, possibly facilitating a gain in the rate of transfer[5] with increases up to 80%.[2]
Final edits (ASSIGNMENT 5)- "Exoelectrogen"
Extracellular electron transport mechanisms
[edit]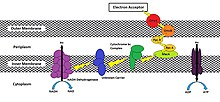

Reduced oxidoreductase enzymes at the extracellular membrane have been shown to use the following methods in transferring their electrons to the exogenous final acceptor: direct contact, shuttling via excreted mediators, iron chelating agents[3], through a conductive biofilm, and through conductive pili (Figure 2). However, the possibility exists that these methods are not mutually exclusive[1], and the method(s) used may depend on environmental conditions. Under low microbial population densities, usage of electron shuttles and chelators synthesized by the exoelectrogen may be energetically costly due to insufficient concentrations of such molecules required for recovery and reuse[3]. Under these circumstances, direct transfer would be favored; however, energy benefits would outweigh energy demands using shuttlers/chelators when the microbial community is of sufficient size.
Direct reduction of an exogenous acceptor is done through contact between the cell’s oxidoreductases and the terminal electron acceptor (i.e. an electrode or external metal compound). While these proteins are diverse (taking on both membrane-bound or soluble forms), their common locations in the outer membrane or periplasm in Gram-negative and Gram-positive bacteria provide intimate contact for electron transfer[4].
Additionally, the presence of electron shuttling molecules dramatically increases the direct transfer rate by up to 80%[2]. As an example, in Shewanella oneidensis MR-1, transport is characterized through a series of redox and structural proteins[5] extending from the cytoplasmic membrane to the outer cell surface (similar to Figure 1). Flavins are secreted which are thought to bridge the “gap” between cell surface protein(s) and the external metal, which may alleviate the need for immediate contact and facilitate transfer at a distance[3]. Furthermore, since cytochromes generally recognize specific surfaces on the substrate metal[4], soluble flavins may act as a universal bridge allowing for electron donation to a variety of different metal shapes and sizes[2], which may be useful in microbial fuel cell applications. Flavins have also been hypothesized to bind to terminal electron transfer proteins as co-factors to increase oxidation rates[5].
In the case of Geobacter sulferreducens, the electron carrier riboflavin is used; however, the electron carrier is not entirely freely soluble and can be loosely bound in the culture's biofilm, resulting in high conductivity. Furthermore, G. sulferreducens produces electrically conductive pili (nanowires) with OmcS oxidoreductase enzymes embedded on its surface[6], demonstrating the usage of multiple exoelectrogenic transfer methods.
In iron chelation, insoluble ferric oxide compounds are solubilized in aqueous solutions. As bioavailability of iron is scarce, many microbes secrete iron chelating compounds to solubilize, uptake, and sequester iron for various cellular processes. Certain exoelectrogens have shown capability of using such compounds for electron transport by solubilizing iron extracellularly[4], and delivering it to the cell surface or within the cell. The components used in each pathway are phylogenetically diverse[5], thus some chelating agents may reduce iron outside the cell acting as electron shuttles, while others may deliver iron to the cell for membrane bound reduction[4].
Kevinxchan (talk) 23:04, 19 November 2017 (UTC)
- ^ a b c Lovley, Derek R (December 2008). "The microbe electric: conversion of organic matter to electricity". Current Opinion in Biotechnology. 19 (6): 564–571. doi:10.1016/j.copbio.2008.10.005.
- ^ a b c d Baron, Daniel; LaBelle, Edward; Coursolle, Dan; Gralnick, Jeffrey A.; Bond, Daniel R. (16 October 2009). "Electrochemical Measurement of Electron Transfer Kinetics by MR-1". Journal of Biological Chemistry. 284 (42): 28865–28873. doi:10.1074/jbc.M109.043455.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d Nevin, Kelly P.; Lovley, Derek R. (March 2002). "Mechanisms for Fe(III) Oxide Reduction in Sedimentary Environments". Geomicrobiology Journal. 19 (2): 141–159. doi:10.1080/01490450252864253.
- ^ a b c d e f g Gralnick, Jeffrey A.; Newman, Dianne K. (July 2007). "Extracellular respiration". Molecular Microbiology. 65 (1): 1–11. doi:10.1111/j.1365-2958.2007.05778.x.
- ^ a b c d e Shi, Liang; Dong, Hailiang; Reguera, Gemma; Beyenal, Haluk; Lu, Anhuai; Liu, Juan; Yu, Han-Qing; Fredrickson, James K. (30 August 2016). "Extracellular electron transfer mechanisms between microorganisms and minerals". Nature Reviews Microbiology. 14 (10): 651–662. doi:10.1038/nrmicro.2016.93.
- ^ Leang C.; et al. (2010). "Alignment of the c-Type Cytochrome OmcS along Pili of Geobacter sulfurreducens". Applications of Environmental Microbiology. 76 (12): 4080–4084. doi:10.1128/AEM.00023-10.
