User:Ndurfee/sandbox
Modifications
[edit]While the Simmons-Smith reaction is often discussed in its basic form, a number of modifications to both the zinc catalyst and the added carbon have been proposed.
Furukawa Modification
[edit]The Furukawa Modification involves the replacement of the zinc copper couple with dialkyl zinc, the most active of which was found to be Et2Zn. The modification was proposed in 1968 as a way to turn cationically polymerizable olefins such as vinyl ethers into their respective cyclopropanes.[1] It has also been found to be especially useful for the cyclopropanation of carbohydrates, being far more reproducible than other methods.[2] Like the unmodified reaction, the furukawa-modified reaction is stereospecific, and is often much faster than the unmodified reaction. However, the Et2Zn reagent is pyrophoric, and as such must be handled with care.[3]
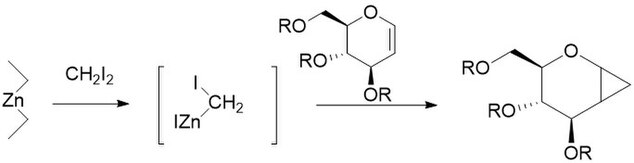
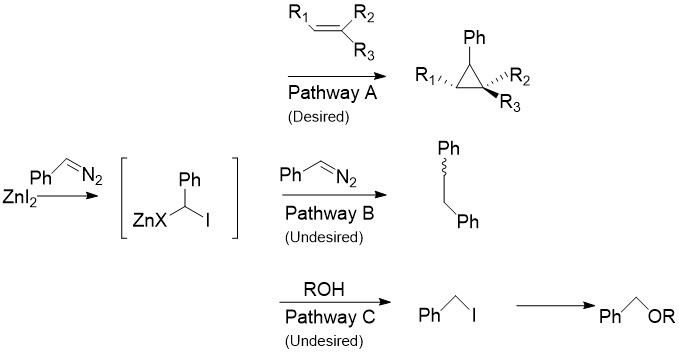
Charette Modification
[edit]The Charette Modification replaces the CH2I2 normally found in the Simmons-Smith reaction with aryldiazo compounds, such as phenyldiazo, in Pathway A.[4] Upon treatment with stoichiometric amounts of Zinc Halide, an organo-zinc compound similar to the carbenoid discussed above is produced. This can react with almost all alkenes and alkynes, including styrenes and alcohols. This is especially useful, as the unmodified simmons smith is known to deprotonate alcohols. Unfortunately, as in Pathway B shown the intermediate can also react with the starting diazo compound, giving cis or trans 1,2-diphenylethene. Additionally, the intermediate can react with alcohols to produce iodophenylmethane, which can further undergo an SN2 reaction to produce ROCHPh, as in Pathway C.
Non-Zinc Reagents.
[edit]Although un-commonly used, Simmons-Smith Reagents that display similar reactive properties to those of Zinc have been prepared from Aluminum and Samarium compounds reacting with CH2I2.[5] The aluminum compound, i-Bu3Al, cyclopropanates geraniol at the 6 position near stereospecifically, while the Samarium compound, Sm/Hg, will cyclopropanate at the 2 position with complete stereospecificity. The specificity of these compounds allow cyclopropanes to be placed in poly-unsaturated systems that zinc-based carbenoids will fully cyclopropanate. However, both reactions require high amounts of the starting metal compound, and Sm/Hg must be activated with the highly toxic HgCl2.
References
[edit]- ^ Furukawa, J; Kawabata, N; Nishimura, J (1968). ""Synthesis of cyclopropanes by the reaction of olefins with dialkylzinc and methylene iodide"". Tetrahedron. 24 (1): 53–58. doi:10.1016/0040-4020(68)89007-6.
- ^ Halton, B (2000). Advances in Strained and Interesting Organic Molecules, Volume 8. Stamford, Ct: Press Inc. p. 115. ISBN 0-7623-0631-9.
- ^ "Diethyl Zinc MSDS" (PDF). Retrieved 10 May 2017.
- ^ Lévesque, Éric; Goudreau, Sébastien R.; B. Charette, André B. (2014). "Improved Zinc-Catalyzed Simmons–Smith Reaction: Access to Various 1,2,3-Trisubstituted Cyclopropanes". Organic Letters. 16 (5): 1490–1493. doi:10.1021/ol500267w. PMID 24555697.
- ^ Roger, Adams (2001). Organic Reactions Vol 58. New York: Wiley, J. pp. 9–10. ISBN 0-471-10590-2.
