Ynol

In chemistry, an ynol (or alkynol) is an alkyne with a hydroxyl group affixed to one of the two carbons composing the triple bond. The deprotonated anions of ynols are known as ynolates. An ynol with hydroxyl groups on both sides of its triple bond is known as an ynediol; only one ynediol, acetylenediol, can exist.
Ynolates

Ynolates are chemical compounds with a negatively charged oxygen attached to an alkyne functionality.[1] They were first synthesized in 1975 by Schöllkopf and Hoppe via the n-butyllithium fragmentation of 3,4-diphenylisoxazole.[2]
Synthetically, they behave as ketene precursors or synthons.
Ynol–ketene tautomerism
Ynols can interconvert with ketenes. The ynol form is usually unstable, does not survive long, and changes into the ketene. This is because oxygen is more electronegative than carbon and thus forms stronger bonds. For instance, ethynol quickly interconverts with ethenone:
| Ynol-ketene tautomeres | |
|---|---|
 |
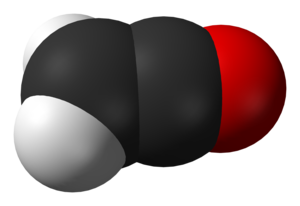
|
| Ethynol | Ethenone |
See also
References
- ^ M. Shindo (2007). "Synthetic uses of ynolates". Tetrahedron. 63 (1): 10–36. doi:10.1016/j.tet.2006.09.013.
- ^ U. Schöllkopf and I. Hoppe (1975). "Lithium Phenylethynolate and Its Reaction with Carbonyl Compounds to Give β-Lactones". Angewandte Chemie International Edition in English. 14 (11): 765. doi:10.1002/anie.197507651.
