Zincke nitration
| Zincke nitration | |
|---|---|
| Named after | Theodor Zincke |
| Reaction type | Substitution reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000413 |
The Zincke nitration is an organic reaction in which a bromine substituent of a phenol or cresol is replaced by a nitro group by treatment with nitrous acid or sodium nitrite.[1] The reaction is a manifestation of nucleophilic aromatic substitution. The reaction is named after Theodor Zincke.
Two examples:[2]
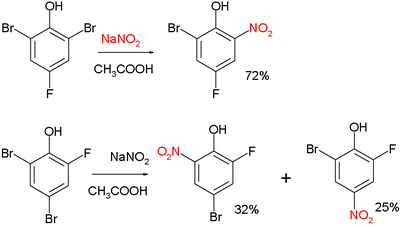
and:[3]

The Zincke nitration should not be confused with the Zincke–Suhl reaction or the Zincke reaction.
See also
References
- ^ Zincke, Th., J. Prakt. Chem. 61, 561–567 (1900) and correction at J. Prakt. Chem. 63, 183 ff (1900).
- ^ The Nitration of Brominated Fluorophenols by the Zincke Method L. Chas. Raiford and Arthur L. LeRosen J. Am. Chem. Soc.; 1944; 66(11) pp 1872–73; doi:10.1021/ja01239a020
- ^ Behavior of Mixed Halogenated Phenols in the Zincke Method of Nitration L. Chas. Raiford and Glen R. Miller J. Am. Chem. Soc.; 1933; 55(5) pp 2125–31; doi:10.1021/ja01332a059
