Casopitant
Appearance
(Redirected from Zunrisa)
 | |
| Clinical data | |
|---|---|
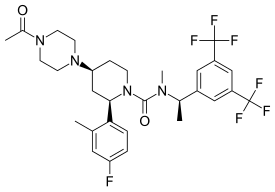
| Other names | GW679769; GW-679769; (2R,4S)-4-(4-Acetylpiperazin-1-yl)-N-{(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethyl}-2-(4-fluoro-2-methylphenyl)-N-methylpiperidine-1-carboxamide |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C30H35F7N4O2 |
| Molar mass | 616.625 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Casopitant (INN),[1]: 208 former tentative trade names Rezonic (U.S.) and Zunrisa (Europe), is an NK1 receptor antagonist which was undergoing research for the treatment of chemotherapy-induced nausea and vomiting.[2][3] It was under development by GlaxoSmithKline.[2] In July 2008, the company filed a marketing authorisation application with the European Medicines Agency. The application was withdrawn and development was discontinued in September 2009 because GlaxoSmithKline decided that further safety assessment was necessary.[2][4] However, a 2022 review listed casopitant as under development as a potential novel antidepressant for the treatment of major depressive disorder, with a phase 2 clinical trial having been completed.[5]
References
[edit]- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List56" (PDF). World Health Organization. 2006. Retrieved 17 November 2016.
- ^ a b c Name, Drug (25 December 2021). "Casopitant". AdisInsight. Retrieved 21 August 2022.
- ^ Lohr L (2008). "Chemotherapy-induced nausea and vomiting". Cancer Journal. 14 (2): 85–93. doi:10.1097/PPO.0b013e31816a0f07. PMID 18391612. S2CID 43224257.
- ^ "GlaxoSmithKline withdraws its marketing authorisation application for Zunrisa" (PDF). London: EMEA. 13 October 2009. Archived from the original (PDF) on 15 October 2009. Retrieved 21 December 2009.
- ^ Sakurai H, Yonezawa K, Tani H, Mimura M, Bauer M, Uchida H (July 2022). "Novel Antidepressants in the Pipeline (Phase II and III): A Systematic Review of the US Clinical Trials Registry". Pharmacopsychiatry. 55 (4): 193–202. doi:10.1055/a-1714-9097. PMC 9259184. PMID 35045580.
