Oxidative coupling
Oxidative coupling in chemistry is a coupling reaction of two molecular entities through an oxidative process. Usually oxidative couplings are catalysed by a transition metal complex like in classical cross-coupling reactions, although the underlying mechanism is different due to the oxidation process that requires an external (or internal) oxidant.[1][2] Many such couplings utilize dioxygen as the stoichiometric oxidant but proceed by electron transfer.[3]
C-C Couplings
[edit]Many oxidative couplings generate new C-C bonds. Early examples involve coupling of terminal alkynes:[4]
- 2 RC≡CH + 2 Cu(I) → RC≡C-C≡CR + 2 Cu + 2 H+
Aromatic coupling
[edit]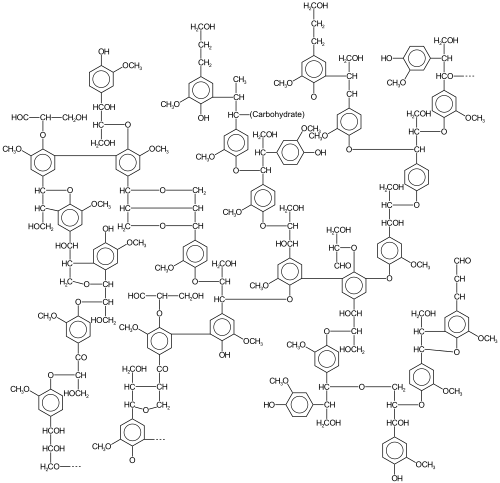
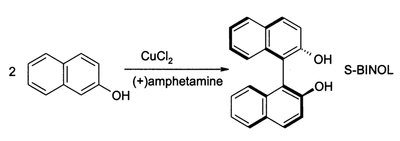
In oxidative aromatic coupling the reactants are electron-rich aromatic compounds. Typical substrates are phenols and typical catalysts are copper and iron compounds and enzymes,[6] although Scholl demonstrated that high heat and a Lewis acid suffice. The first reported synthetic application dates back to 1868 with Julius Löwe and the synthesis of ellagic acid by heating gallic acid with arsenic acid or silver oxide.[7] Another reaction is the synthesis of 1,1'-Bi-2-naphthol from 2-naphthol by iron chloride, discovered in 1873 by Alexander Dianin[8] (S)-BINOL can be prepared directly from an asymmetric oxidative coupling of 2-naphthol with copper(II) chloride.[9]
Coupling of methane
[edit]Coupling reactions involving methane are highly sought, related to C1 chemistry because C2 derivatives are far more valuable than methane. The oxidative coupling of methane gives ethylene:[10][11]
- 2CH
4 + O
2 → C
2H
4 + 2H
2O
Other oxidative couplings
[edit]
The oxygen evolution reaction entails, in effect, the oxidative coupling of water molecules to give O2.
References
[edit]- ^ Oxidative Cross-Coupling Reactions. Aiwen Lei, Wei Shi, Chao Liu, Wei Liu, Hua Zhang, Chuan He, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany (2017). doi:10.1002/9783527680986
- ^ Ignacio Funes-Ardoiz; Feliu Maseras (2018). "Oxidative Coupling Mechanisms: Current State of Understanding". ACS Catalysis. 8 (2): 1161–1172. doi:10.1021/acscatal.7b02974.
- ^ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). doi:10.1351/goldbook
- ^ Alison E. Wendlandt; Alison M. Suess; Shannon S. Stahl (2011). "Copper-Catalyzed Aerobic Oxidative C-H Functionalizations: Trends and Mechanistic Insights". Angew. Chem. Int. Ed. 50 (47): 11062–11087. doi:10.1002/anie.201103945. PMID 22034061.
- ^ Lebo, Stuart E. Jr.; Gargulak, Jerry D.; McNally, Timothy J. (2001). "Lignin". Kirk-Othmer Encyclopedia of Chemical Technology. Kirk‑Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. doi:10.1002/0471238961.12090714120914.a01.pub2. ISBN 0-471-23896-1. Retrieved 2007-10-14.
- ^ Grzybowski, M., Skonieczny, K., Butenschön, H. and Gryko, D. T. (2013), Comparison of Oxidative Aromatic Coupling and the Scholl Reaction Angew. Chem. Int. Ed., 52: 9900–9930. doi:10.1002/anie.201210238
- ^ Löwe, Zeitschrift für Chemie, 1868, 4, 603
- ^ A. P. Dianin, Zh. Russ. Fiz.-Khim. O-va. 1874 , 183
- ^ Brussee, J.; Jansen, A. C. A. (1983). "A highly stereoselective synthesis of S-(−)-[1,1′-binaphthalene]-2,2′-diol". Tetrahedron Letters. 24 (31): 3261–3262. doi:10.1016/S0040-4039(00)88151-4.
- ^ Zhang, Q. (2003). "Recent Progress in Direct Partial Oxidation of Methane to Methanol". J. Natural Gas Chem. 12: 81–89.
- ^ Olah, G., Molnar, A. "Hydrocarbon Chemistry" John Wiley & Sons, New York, 2003. ISBN 978-0-471-41782-8.
