Disufenton sodium: Difference between revisions
elaborating on compound type with reference |
→Research: Adding information about brain tumor and cancer use |
||
| Line 55: | Line 55: | ||
==Research== |
==Research== |
||
Disufenton sodium was under development at the drug company [[AstraZeneca]]. A 2005 phase-3 clinical trial<ref name="DoiNEJMoa">{{cite journal | vauthors = Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, Grotta J, Lyden P, Shuaib A, Hårdemark HG, Wasiewski WW | display-authors = 6 | title = NXY-059 for acute ischemic stroke | journal = The New England Journal of Medicine | volume = 354 | issue = 6 | pages = 588–600 | date = February 2006 | pmid = 16467546 | doi = 10.1056/NEJMoa052980 }}</ref><ref>{{cite journal | vauthors = Lees KR, Davalos A, Davis SM, Diener HC, Grotta J, Lyden P, Shuaib A, Ashwood T, Hardemark HG, Wasiewski W, Emeribe U, Zivin JA | display-authors = 6 | title = Additional outcomes and subgroup analyses of NXY-059 for acute ischemic stroke in the SAINT I trial | journal = Stroke | volume = 37 | issue = 12 | pages = 2970–8 | date = December 2006 | pmid = 17068304 | doi = 10.1161/01.STR.0000249410.91473.44 | doi-access = free }}</ref> called "SAINT-1" reported some efficacy in the acute treatment of [[ischemia]] injury due to [[stroke]]. However, a 2006 attempt to repeat this trial indicated no significant activity. After ruling out other causes, the authors tentatively attributed the positive results in the first trial to "chance".<ref name="DoiNEJMoa" /> AstraZeneca then terminated the development programme.<ref>{{cite web | url = http://investors.renovis.com/phoenix.zhtml?c=148297&p=irol-newsArticle&t=Regular&id=921678& | title = Renovis: Press Release| archive-url = https://web.archive.org/web/20061028094321/http://investors.renovis.com/phoenix.zhtml?c=148297&p=irol-newsArticle&t=Regular&id=921678& | archive-date=October 28, 2006 }}</ref> |
Disufenton sodium was under development at the drug company [[AstraZeneca]]. A 2005 phase-3 clinical trial<ref name="DoiNEJMoa">{{cite journal | vauthors = Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, Grotta J, Lyden P, Shuaib A, Hårdemark HG, Wasiewski WW | display-authors = 6 | title = NXY-059 for acute ischemic stroke | journal = The New England Journal of Medicine | volume = 354 | issue = 6 | pages = 588–600 | date = February 2006 | pmid = 16467546 | doi = 10.1056/NEJMoa052980 }}</ref><ref>{{cite journal | vauthors = Lees KR, Davalos A, Davis SM, Diener HC, Grotta J, Lyden P, Shuaib A, Ashwood T, Hardemark HG, Wasiewski W, Emeribe U, Zivin JA | display-authors = 6 | title = Additional outcomes and subgroup analyses of NXY-059 for acute ischemic stroke in the SAINT I trial | journal = Stroke | volume = 37 | issue = 12 | pages = 2970–8 | date = December 2006 | pmid = 17068304 | doi = 10.1161/01.STR.0000249410.91473.44 | doi-access = free }}</ref> called "SAINT-1" reported some efficacy in the acute treatment of [[ischemia]] injury due to [[stroke]]. However, a 2006 attempt to repeat this trial indicated no significant activity. After ruling out other causes, the authors tentatively attributed the positive results in the first trial to "chance".<ref name="DoiNEJMoa" /> AstraZeneca then terminated the development programme.<ref>{{cite web | url = http://investors.renovis.com/phoenix.zhtml?c=148297&p=irol-newsArticle&t=Regular&id=921678& | title = Renovis: Press Release| archive-url = https://web.archive.org/web/20061028094321/http://investors.renovis.com/phoenix.zhtml?c=148297&p=irol-newsArticle&t=Regular&id=921678& | archive-date=October 28, 2006 }}</ref> |
||
Disufenton sodium has been researched as a potential treatment for use in brain tumors and cancers, including [[diffuse intrinsic pontine glioma]] (DIPG)<ref name="Thomas Smith Saunders Zalles p. ">{{cite journal | last=Thomas | first=Lincy | last2=Smith | first2=Nataliya | last3=Saunders | first3=Debra | last4=Zalles | first4=Michelle | last5=Gulej | first5=Rafal | last6=Lerner | first6=Megan | last7=Fung | first7=Kar-Ming | last8=Carcaboso | first8=Angel M. | last9=Towner | first9=Rheal A. | title=OKlahoma Nitrone-007: novel treatment for diffuse intrinsic pontine glioma | journal=Journal of Translational Medicine | publisher=Springer Science and Business Media LLC | volume=18 | issue=1 | date=November 10, 2020 | issn=1479-5876 | doi=10.1186/s12967-020-02593-5}}</ref><ref name="Healio 2021">{{cite web | title=FDA grants fast track status to OKN-007 for diffuse intrinsic pontine glioma | website=Healio | date=March 3, 2021 | url=https://www.healio.com/news/hematology-oncology/20210303/fda-grants-fast-track-status-to-okn007-for-diffuse-intrinsic-pontine-glioma | access-date=February 5, 2022}}</ref> and [[glioblastoma]]<ref name="Battiste 2020">{{cite journal | last=Battiste | first=James D. | last2=Ikeguchi | first2=Alexandra | last3=Woo | first3=Sukyung | last4=Sharan | first4=Satish | last5=Zhao | first5=Yan D. | last6=Cohoon | first6=Andrew | last7=Sung | first7=Sarah | last8=Wright | first8=Deborah | last9=Teague | first9=April M. | last10=Jensen | first10=Randy L. | last11=Kim | first11=Eun Ha | last12=Yang | first12=Won S. | last13=Towner | first13=Rheal A. | title=Phase Ib clinical trial of OKN-007 in recurrent malignant glioma. | journal=Journal of Clinical Oncology | publisher=American Society of Clinical Oncology (ASCO) | volume=38 | issue=15_suppl | date=May 20, 2020 | issn=0732-183X | doi=10.1200/jco.2020.38.15_suppl.2538 | pages=2538–2538}}</ref><ref name="Zalles 2021">{{cite journal | last=Zalles | first=Michelle | last2=Smith | first2=Nataliya | last3=Saunders | first3=Debra | last4=Lerner | first4=Megan | last5=Fung | first5=Kar‐Ming | last6=Battiste | first6=James | last7=Towner | first7=Rheal A. | title=A tale of two multi‐focal therapies for glioblastoma: An antibody targeting ELTD1 and nitrone‐based OKN‐007 | journal=Journal of Cellular and Molecular Medicine | publisher=Wiley | volume=26 | issue=2 | date=December 14, 2021 | issn=1582-1838 | doi=10.1111/jcmm.17133 | pages=570–582}}</ref>. |
|||
==Chemistry== |
==Chemistry== |
||
Revision as of 04:15, 5 February 2022
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
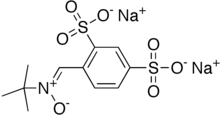
| Formula | C11H13NNa2O7S2 |
| Molar mass | 381.32 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Disufenton sodium (Cerovive, NXY-059, HPN-07)[1] is a free radical trapping nitrone-based antioxidant compound that has been under development for several medical conditions.[2][3]
Research
Disufenton sodium was under development at the drug company AstraZeneca. A 2005 phase-3 clinical trial[4][5] called "SAINT-1" reported some efficacy in the acute treatment of ischemia injury due to stroke. However, a 2006 attempt to repeat this trial indicated no significant activity. After ruling out other causes, the authors tentatively attributed the positive results in the first trial to "chance".[4] AstraZeneca then terminated the development programme.[6]
Disufenton sodium has been researched as a potential treatment for use in brain tumors and cancers, including diffuse intrinsic pontine glioma (DIPG)[7][8] and glioblastoma[9][10].
Chemistry
Disufenton sodium is the disulfonyl derivative of the neuroprotective nitrone spin trap phenylbutylnitrone or "PBN". PBN and its derivatives hydrolyze and oxidize in vitro to form respectively MNP-OH (AKA, NtBHA) and its parent spin-trap MNP.
References
- ^ Varela-Nieto, Isabel; Murillo-Cuesta, Silvia; Rodríguez-de la Rosa, Lourdes; Oset-Gasque, María Jesús; Marco-Contelles, José (September 1, 2021). "Use of Radical Oxygen Species Scavenger Nitrones to Treat Oxidative Stress-Mediated Hearing Loss: State of the Art and Challenges". Frontiers in Cellular Neuroscience. 15. Frontiers Media SA. doi:10.3389/fncel.2021.711269. ISSN 1662-5102.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Zhao, Zonghang; Cheng, Mingshan; Maples, Kirk R.; Ma, Jing Ying; Buchan, Alastair M. (2001). "NXY-059, a novel free radical trapping compound, reduces cortical infarction after permanent focal cerebral ischemia in the rat". Brain Research. 909 (1–2). Elsevier BV: 46–50. doi:10.1016/s0006-8993(01)02618-x. ISSN 0006-8993.
- ^ Choi, Seong Hee; Choi, Chul-Hee (December 20, 2015). "Noise-Induced Neural Degeneration and Therapeutic Effect of Antioxidant Drugs". Journal of Audiology and Otology. 19 (3). The Korean Audiological Society: 111–119. doi:10.7874/jao.2015.19.3.111. ISSN 2384-1621.
- ^ a b Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, et al. (February 2006). "NXY-059 for acute ischemic stroke". The New England Journal of Medicine. 354 (6): 588–600. doi:10.1056/NEJMoa052980. PMID 16467546.
- ^ Lees KR, Davalos A, Davis SM, Diener HC, Grotta J, Lyden P, et al. (December 2006). "Additional outcomes and subgroup analyses of NXY-059 for acute ischemic stroke in the SAINT I trial". Stroke. 37 (12): 2970–8. doi:10.1161/01.STR.0000249410.91473.44. PMID 17068304.
- ^ "Renovis: Press Release". Archived from the original on October 28, 2006.
- ^ Thomas, Lincy; Smith, Nataliya; Saunders, Debra; Zalles, Michelle; Gulej, Rafal; Lerner, Megan; Fung, Kar-Ming; Carcaboso, Angel M.; Towner, Rheal A. (November 10, 2020). "OKlahoma Nitrone-007: novel treatment for diffuse intrinsic pontine glioma". Journal of Translational Medicine. 18 (1). Springer Science and Business Media LLC. doi:10.1186/s12967-020-02593-5. ISSN 1479-5876.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "FDA grants fast track status to OKN-007 for diffuse intrinsic pontine glioma". Healio. March 3, 2021. Retrieved February 5, 2022.
- ^ Battiste, James D.; Ikeguchi, Alexandra; Woo, Sukyung; Sharan, Satish; Zhao, Yan D.; Cohoon, Andrew; Sung, Sarah; Wright, Deborah; Teague, April M.; Jensen, Randy L.; Kim, Eun Ha; Yang, Won S.; Towner, Rheal A. (May 20, 2020). "Phase Ib clinical trial of OKN-007 in recurrent malignant glioma". Journal of Clinical Oncology. 38 (15_suppl). American Society of Clinical Oncology (ASCO): 2538–2538. doi:10.1200/jco.2020.38.15_suppl.2538. ISSN 0732-183X.
- ^ Zalles, Michelle; Smith, Nataliya; Saunders, Debra; Lerner, Megan; Fung, Kar‐Ming; Battiste, James; Towner, Rheal A. (December 14, 2021). "A tale of two multi‐focal therapies for glioblastoma: An antibody targeting ELTD1 and nitrone‐based OKN‐007". Journal of Cellular and Molecular Medicine. 26 (2). Wiley: 570–582. doi:10.1111/jcmm.17133. ISSN 1582-1838.
