Antifreeze protein: Difference between revisions
ref reformatting |
more ref reformatting |
||
| Line 55: | Line 55: | ||
| image = PDB 1l1i EBI.jpg |
| image = PDB 1l1i EBI.jpg |
||
| width = |
| width = |
||
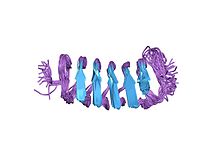
| caption = Structure of the ''[[Tenebrio molitor]]'' beta-helical antifreeze protein<ref name="pmid11969412">{{cite journal |author=Daley ME, Spyracopoulos L, Jia Z, Davies PL, Sykes BD |title=Structure and dynamics of a beta-helical antifreeze protein |journal=Biochemistry |volume=41 |issue=17 |pages=5515–25 |year=2002 |month=April |pmid=11969412 |doi= 10.1021/bi0121252 |
| caption = Structure of the ''[[Tenebrio molitor]]'' beta-helical antifreeze protein<ref name="pmid11969412">{{cite journal | author = Daley ME, Spyracopoulos L, Jia Z, Davies PL, Sykes BD | title = Structure and dynamics of a beta-helical antifreeze protein | journal = Biochemistry | volume = 41 | issue = 17 | pages = 5515–25 | year = 2002 | month = April | pmid = 11969412 | doi = 10.1021/bi0121252 }}</ref> |
||
| Pfam = PF02420 |
| Pfam = PF02420 |
||
| Pfam_clan = |
| Pfam_clan = |
||
| Line 73: | Line 73: | ||
| image = PDB 1m8n EBI.jpg |
| image = PDB 1m8n EBI.jpg |
||
| width = |
| width = |
||
| caption = Structure of ''Choristoneura fumiferana'' (spruce budworm) beta-helical antifreeze protein<ref name="pmid12105229">{{cite journal |author=Leinala EK, Davies PL, Doucet D, Tyshenko MG, Walker VK, Jia Z |title=A beta-helical antifreeze protein isoform with increased activity. Structural and functional insights |journal=J. Biol. Chem. |volume=277 |issue=36 |pages=33349–52 |year=2002 |month=September |pmid=12105229 |doi=10.1074/jbc.M205575200 |
| caption = Structure of ''Choristoneura fumiferana'' (spruce budworm) beta-helical antifreeze protein<ref name="pmid12105229">{{cite journal | author = Leinala EK, Davies PL, Doucet D, Tyshenko MG, Walker VK, Jia Z | title = A beta-helical antifreeze protein isoform with increased activity. Structural and functional insights | journal = J. Biol. Chem. | volume = 277 | issue = 36 | pages = 33349–52 | year = 2002 | month = September | pmid = 12105229 | doi = 10.1074/jbc.M205575200 }}</ref> |
||
| Pfam = PF05264 |
| Pfam = PF05264 |
||
| Pfam_clan = |
| Pfam_clan = |
||
| Line 90: | Line 90: | ||
===Sea ice organisms AFPs=== |
===Sea ice organisms AFPs=== |
||
AFPs were also found in microorganisms living in [[sea ice]]. The [[diatom]]s ''Fragilariopsis cylindrus'' and ''F. curta'' play a key role in polar sea ice communities, dominating the assemblages of both platelet layer and within pack ice. AFPs are widespread in these species, and the presence of AFP [[genes]] as a multigene family indicates the importance of this group for the genus ''Fragilariopsis''.<ref>Bayer-Giraldi |
AFPs were also found in microorganisms living in [[sea ice]]. The [[diatom]]s ''Fragilariopsis cylindrus'' and ''F. curta'' play a key role in polar sea ice communities, dominating the assemblages of both platelet layer and within pack ice. AFPs are widespread in these species, and the presence of AFP [[genes]] as a multigene family indicates the importance of this group for the genus ''Fragilariopsis''.<ref name="pmid20105220">{{cite journal | author = Bayer-Giraldi M, Uhlig C, John U, Mock T, Valentin K | title = Antifreeze proteins in polar sea ice diatoms: diversity and gene expression in the genus Fragilariopsis | journal = Environ. Microbiol. | volume = 12 | issue = 4 | pages = 1041–52 | year = 2010 | month = April | pmid = 20105220 | doi = 10.1111/j.1462-2920.2009.02149.x }}</ref> AFPs identified in ''F. cylindrus'' belong to an AFP family which is represented in different taxa and can be found in other organisms related to sea ice (''[[Colwellia]]'' spp., ''[[Navicula glaciei]]'', ''[[Chaetoceros neogracile]]'' and ''[[Stephos longipes]] and Leucosporidium antarcticum''<ref name="pmid17651136">{{cite journal | author = Raymond JA, Fritsen C, Shen K | title = An ice-binding protein from an Antarctic sea ice bacterium | journal = FEMS Microbiol. Ecol. | volume = 61 | issue = 2 | pages = 214–21 | year = 2007 | month = August | pmid = 17651136 | doi = 10.1111/j.1574-6941.2007.00345.x }}</ref><ref>Kiko, R. (2010): Acquisition of freeze protection in a sea-ice crustacean through horizontal gene transfer? Polar Biology (33) 543-556.</ref>) and Antarctic inland ice bacteria ([[Flavobacteriaceae]],<ref name="pmid18622572">{{cite journal | author = Raymond JA, Christner BC, Schuster SC | title = A bacterial ice-binding protein from the Vostok ice core | journal = Extremophiles | volume = 12 | issue = 5 | pages = 713–7 | year = 2008 | month = September | pmid = 18622572 | doi = 10.1007/s00792-008-0178-2 }}</ref><ref>http://pnwfungi.org/pdf_files/manuscripts_volume_5/naf20105/naf2010514.pdf</ref> as well as in cold-tolerant fungi (''[[Typhula ishikariensis]]'', ''[[Lentinula edodes]]'' and ''[[Flammulina populicola]]''.<ref>Hoshino, T., Kiriaki, M., Ohgiya, S., Fujiwara, M., Kondo, H., Nishimiya, Y., et al. (2003) Antifreeze proteins from snow mold fungi. Can J Bot 81: 1175–1181.</ref><ref name="pmid19121299">{{cite journal | author = Raymond JA, Janech MG | title = Ice-binding proteins from enoki and shiitake mushrooms | journal = Cryobiology | volume = 58 | issue = 2 | pages = 151–6 | year = 2009 | month = April | pmid = 19121299 | doi = 10.1016/j.cryobiol.2008.11.009 }}</ref>) |
||
== Evolution == |
== Evolution == |
||
The remarkable diversity and distribution of AFPs suggest the different types evolved recently in response to sea level [[glaciation]] occurring 1-2 million years ago in the Northern hemisphere and 10-30 million years ago in Antarctica. This independent development of similar adaptations is referred to as [[convergent evolution]].<ref name="Fletcher2001" /> There are two reasons why many types of AFPs are able to carry out the same function despite their diversity: |
The remarkable diversity and distribution of AFPs suggest the different types evolved recently in response to sea level [[glaciation]] occurring 1-2 million years ago in the Northern hemisphere and 10-30 million years ago in Antarctica. This independent development of similar adaptations is referred to as [[convergent evolution]].<ref name="Fletcher2001" /> There are two reasons why many types of AFPs are able to carry out the same function despite their diversity: |
||
# Although ice is uniformly composed of [http://www.bell-labs.com/news/1999/january/12/ice1h.jpg oxygen and hydrogen], it has many different surfaces exposed for binding. Different types of AFPs may interact with different surfaces. |
# Although ice is uniformly composed of [http://www.bell-labs.com/news/1999/january/12/ice1h.jpg oxygen and hydrogen], it has many different surfaces exposed for binding. Different types of AFPs may interact with different surfaces. |
||
# Although the five types of AFPs differ in their [[primary sequence]] of amino acids, when each folds into a functioning protein, they may share similarities in their three dimensional or [[tertiary structure]] that facilitates the same interactions with ice.<ref name="Fletcher2001" /><ref> |
# Although the five types of AFPs differ in their [[primary sequence]] of amino acids, when each folds into a functioning protein, they may share similarities in their three dimensional or [[tertiary structure]] that facilitates the same interactions with ice.<ref name="Fletcher2001" /><ref name="pmid9108061">{{cite journal | author = Chen L, DeVries AL, Cheng CH | title = Convergent evolution of antifreeze glycoproteins in Antarctic notothenioid fish and Arctic cod | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 94 | issue = 8 | pages = 3817–22 | year = 1997 | month = April | pmid = 9108061 | pmc = 20524 | doi = }}</ref> |
||
== Mechanisms of action == |
== Mechanisms of action == |
||
AFPs are thought to inhibit growth by an [[adsorption]]–inhibition mechanism.<ref name="Raymond and DeVries1977">{{cite journal | |
AFPs are thought to inhibit growth by an [[adsorption]]–inhibition mechanism.<ref name="Raymond and DeVries1977">{{cite journal | author = Raymond JA, DeVries AL | title = Adsorption inhibition as a mechanism of freezing resistance in polar fishes | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 74 | issue = 6 | pages = 2589–93 | year = 1977 | month = June | pmid = 267952 | pmc = 432219 | doi = 10.1073/pnas.74.6.2589 }}</ref> They adsorb to non[[basal plane]]s of ice, inhibiting thermodynamically favored ice growth.<ref name="Raymond1989">{{cite journal | author = Raymond JA, Wilson P, DeVries AL | title = Inhibition of growth of nonbasal planes in ice by fish antifreezes | journal = Proc. Natl. Acad. Sci. U.S.A. | volume = 86 | issue = 3 | pages = 881–5 | year = 1989 | month = February | pmid = 2915983 | pmc = 286582 | doi = 10.1073/pnas.86.3.881 }}</ref> The presence of a flat, rigid surface in some AFPs seems to facilitate its interaction with ice via [[Van der Waals force]] surface complementarity.<ref name="Yang1998">{{cite journal | author = Yang DS, Hon WC, Bubanko S, Xue Y, Seetharaman J, Hew CL, Sicheri F | title = Identification of the ice-binding surface on a type III antifreeze protein with a "flatness function" algorithm | journal = Biophys. J. | volume = 74 | issue = 5 | pages = 2142–51 | year = 1998 | month = May | pmid = 9591641 | pmc = 1299557 | doi = 10.1016/S0006-3495(98)77923-8 }}</ref> |
||
== Binding to ice == |
== Binding to ice == |
||
Normally, ice crystals grown in solution only exhibit the basal (0001) and prism faces (1010), and appear as round and flat discs.<ref name="Jorov2004" /> However, it appears the presence of AFPs exposes other faces. It now appears the ice surface 2021 is the preferred binding surface, at least for AFP type I.<ref name="Knight1991"> |
Normally, ice crystals grown in solution only exhibit the basal (0001) and prism faces (1010), and appear as round and flat discs.<ref name="Jorov2004" /> However, it appears the presence of AFPs exposes other faces. It now appears the ice surface 2021 is the preferred binding surface, at least for AFP type I.<ref name="Knight1991">{{cite journal | author = Knight CA, Cheng CC, DeVries AL | title = Adsorption of alpha-helical antifreeze peptides on specific ice crystal surface planes | journal = Biophys. J. | volume = 59 | issue = 2 | pages = 409–18 | year = 1991 | month = February | pmid = 2009357 | pmc = 1281157 | doi = 10.1016/S0006-3495(91)82234-2 | url = }}</ref> Through studies on type I AFP, ice and AFP were initially thought to interact through hydrogen bonding (Raymond and DeVries, 1977). However, when parts of the protein thought to facilitate this hydrogen bonding were mutated, the hypothesized decrease in antifreeze activity was not observed. Recent data suggest hydrophobic interactions could be the main contributor.<ref name="pmid9688560">{{cite journal | author = Haymet AD, Ward LG, Harding MM, Knight CA | title = Valine substituted winter flounder 'antifreeze': preservation of ice growth hysteresis | journal = FEBS Lett. | volume = 430 | issue = 3 | pages = 301–6 | year = 1998 | month = July | pmid = 9688560 | doi = }}</ref> It is difficult to discern the exact mechanism of binding because of the complex water-ice interface. Currently, attempts to uncover the precise mechanism are being made through use of [[molecular modelling]] programs ([[molecular dynamics]] or the [[Monte Carlo method]]).<ref name="Madura2001" /><ref name="Jorov2004" /> |
||
== Binding mechanism and antifreeze function== |
== Binding mechanism and antifreeze function== |
||
| ⚫ | According to the structure and function study on the antifreeze protein from the fish winter flounder,<ref name=chou>{{cite journal | author = Chou KC | title = Energy-optimized structure of antifreeze protein and its binding mechanism | journal = J. Mol. Biol. | volume = 223 | issue = 2 | pages = 509–17 | year = 1992 | month = January | pmid = 1738160 | doi = | url = }}</ref> the antifreeze mechanism of the type-I AFP molecule was shown to be due to the binding to an ice nucleation structure in a zipper-like fashion through hydrogen bonding of the hydroxyl groups of its four Thr residues to the oxygens along the |
||
According to the structure and function study on the antifreeze protein from the fish winter flounder, |
|||
| ⚫ | <ref name=chou> |
||
<math>[01\overline{1}2]</math> direction in ice lattice, subsequently stopping or retarding the growth of ice pyramidal planes so as to depress the freeze point.<ref name=chou/> |
<math>[01\overline{1}2]</math> direction in ice lattice, subsequently stopping or retarding the growth of ice pyramidal planes so as to depress the freeze point.<ref name=chou/> |
||
| Line 114: | Line 113: | ||
== History == |
== History == |
||
In the 1950s, Canadian scientist Scholander set out to explain how Arctic fish can survive in water colder than the freezing point of their blood. His experiments led him to believe there was “antifreeze” in the blood of Arctic fish.<ref name="Madura2001" /> Then in the late 1960s, animal biologist Arthur DeVries was able to isolate the antifreeze protein through his investigation of Antarctic fish.<ref name="De Vries and Wohlschlag1969">{{cite journal | |
In the 1950s, Canadian scientist Scholander set out to explain how Arctic fish can survive in water colder than the freezing point of their blood. His experiments led him to believe there was “antifreeze” in the blood of Arctic fish.<ref name="Madura2001" /> Then in the late 1960s, animal biologist Arthur DeVries was able to isolate the antifreeze protein through his investigation of Antarctic fish.<ref name="De Vries and Wohlschlag1969">{{cite journal | author = DeVries AL, Wohlschlag DE | title = Freezing resistance in some Antarctic fishes | journal = Science | volume = 163 | issue = 871 | pages = 1073–5 | year = 1969 | month = March | pmid = 5764871 | doi = 10.1126/science.163.3871.1073 }}</ref> These proteins were later called antifreeze glycoproteins (AFGPs) or antifreeze glycopeptides to distinguish them from newly discovered nonglycoprotein biological antifreeze agents (AFPs). DeVries worked with Robert Feeney (1970) to characterize the chemical and physical properties of antifreeze proteins.<ref name="DeVries1970">{{cite journal | author = DeVries AL, Komatsu SK, Feeney RE | title = Chemical and physical properties of freezing point-depressing glycoproteins from Antarctic fishes | journal = J. Biol. Chem. | volume = 245 | issue = 11 | pages = 2901–8 | year = 1970 | month = June | pmid = 5488456 | doi = }}</ref> In 1992, Griffith ''et al.'' documented their discovery of AFP in winter rye leaves. Around the same time, Urrutia, Duman and Knight (1992) documented thermal hysteresis protein in angiosperms. The next year, Duman and Olsen noted AFPs had also been discovered in over 23 species of [[angiosperms]], including ones eaten by humans.<ref name="Duman and Olsen1993">{{cite journal |author = Duman JG, Olsen TM | year = 1993 | month = | title = Thermal hysteresis protein activity in bacteria, fungi and phylogenetically diverse plants | journal = Cryobiology | volume = 30 | issue = 3 | pages = 322–328 | doi = 10.1006/cryo.1993.1031 }}</ref> As well, they reported their presence in fungi and bacteria. |
||
== Name change == |
== Name change == |
||
Recent attempts have been made to relabel antifreeze proteins as ice structuring proteins to more accurately represent their function and to dispose of any assumed negative relation between AFPs and automotive antifreeze, [[ethylene glycol]]. These two things are completely separate entities, and show loose similarity only in their function.<ref> |
Recent attempts have been made to relabel antifreeze proteins as ice structuring proteins to more accurately represent their function and to dispose of any assumed negative relation between AFPs and automotive antifreeze, [[ethylene glycol]]. These two things are completely separate entities, and show loose similarity only in their function.<ref name="pmid12050776">{{cite journal | author = Clarke CJ, Buckley SL, Lindner N | title = Ice structuring proteins - a new name for antifreeze proteins | journal = Cryo Letters | volume = 23 | issue = 2 | pages = 89–92 | year = 2002 | pmid = 12050776 | doi = }}</ref> |
||
== Commercial applications == |
== Commercial applications == |
||
| Line 135: | Line 134: | ||
As well, the [[transgenic]] process of ISP production is widely used in society already. This is how mass amounts of [[insulin]] are made to treat people with [[type I diabetes]] each year. The process does not impact the product; it merely makes production more efficient and prevents the death of many fish who would, otherwise, be killed for the extraction of such protein. |
As well, the [[transgenic]] process of ISP production is widely used in society already. This is how mass amounts of [[insulin]] are made to treat people with [[type I diabetes]] each year. The process does not impact the product; it merely makes production more efficient and prevents the death of many fish who would, otherwise, be killed for the extraction of such protein. |
||
Currently, [[Unilever]] incorporates AFPs into some of its American products, including some [[popsicle]]s and a new line of [[Breyers]] Light Double Churned ice cream bars. In ice cream, AFPs allow the production of very creamy, dense, reduced fat ice cream with fewer additives.<ref>[http://www.nytimes.com/2006/07/26/dining/26cream.html Creamy, Healthier Ice Cream? What’s the Catch?]</ref> They control ice crystal growth brought on by thawing on the loading dock or kitchen table which drastically reduces texture quality.<ref name="Regand2006"> |
Currently, [[Unilever]] incorporates AFPs into some of its American products, including some [[popsicle]]s and a new line of [[Breyers]] Light Double Churned ice cream bars. In ice cream, AFPs allow the production of very creamy, dense, reduced fat ice cream with fewer additives.<ref>[http://www.nytimes.com/2006/07/26/dining/26cream.html Creamy, Healthier Ice Cream? What’s the Catch?]</ref> They control ice crystal growth brought on by thawing on the loading dock or kitchen table which drastically reduces texture quality.<ref name="Regand2006">{{cite journal | author = Regand A, Goff HD | title = Ice recrystallization inhibition in ice cream as affected by ice structuring proteins from winter wheat grass | journal = J. Dairy Sci. | volume = 89 | issue = 1 | pages = 49–57 | year = 2006 | month = January | pmid = 16357267 | doi = 10.3168/jds.S0022-0302(06)72068-9 }}</ref> |
||
In November 2009, the [[Proceedings of the National Academy of Sciences]] published the discovery of a molecule in an Alaskan beetle that behaves like AFPs, but is composed of [[Disaccharide|saccharide]]s and [[fatty acid]]s.<ref name="Walters KR Jr, Serianni AS, Sformo T, Barnes BM, Duman JG 2009 20210–5"/> |
In November 2009, the [[Proceedings of the National Academy of Sciences]] published the discovery of a molecule in an Alaskan beetle that behaves like AFPs, but is composed of [[Disaccharide|saccharide]]s and [[fatty acid]]s.<ref name="Walters KR Jr, Serianni AS, Sformo T, Barnes BM, Duman JG 2009 20210–5"/> |
||
A 2010 study demonstrated the stability of superheated water ice crystals in an AFP solution, showing while the proteins can inhibit freezing, they can also inhibit melting.<ref>{{Cite journal | doi = 10.1016/j.bpj.2009.12.1331 | laysource = Physorg.com | laysummary = http://www.physorg.com/news186669694.html | title = Superheating of Ice in the Presence of Ice Binding Proteins | year = 2010 | last1 = Celik | first1 = Y |
A 2010 study demonstrated the stability of superheated water ice crystals in an AFP solution, showing while the proteins can inhibit freezing, they can also inhibit melting.<ref>{{Cite journal | doi = 10.1016/j.bpj.2009.12.1331 | laysource = Physorg.com | laysummary = http://www.physorg.com/news186669694.html | title = Superheating of Ice in the Presence of Ice Binding Proteins | year = 2010 | last1 = Celik | first1 = Y | last2 = Graham | first2 = LA | last3 = Mok | first3 = YF | last4 = Bar | first4 = M | last5 = Davies | first5 = PL | last6 = Braslavsky | first6 = I | journal = Biophysical Journal | volume = 98 | issue = 3 | pages = 245a}}</ref> |
||
== References == |
== References == |
||
Revision as of 11:18, 27 August 2011
Antifreeze proteins (AFPs) or ice structuring proteins (ISPs) refer to a class of polypeptides produced by certain vertebrates, plants, fungi and bacteria that permit their survival in subzero environments. AFPs bind to small ice crystals to inhibit growth and recrystallization of ice that would otherwise be fatal.[1] There is also increasing evidence that AFPs interact with mammalian cell membranes to protect them from cold damage. This work suggests the involvement of AFPs in cold acclimatization.[2]
Non-colligative properties
Unlike the widely used automotive antifreeze, ethylene glycol, AFPs do not lower freezing point in proportion to concentration. Rather, they work in a noncolligative manner. This allows them to act as an antifreeze at concentrations 1/300th to 1/500th of those of other dissolved solutes. This minimizes their effect on osmotic pressure.[2] The unusual capabilities of AFPs are attributed to their binding ability at specific ice crystal surfaces.[3]
Thermal hysteresis
AFPs create a difference between the melting point and freezing point known as thermal hysteresis. The addition of AFPs at the interface between solid ice and liquid water inhibits the thermodynamically favored growth of the ice crystal. Ice growth is kinetically inhibited by the AFPs covering the water-accessible surfaces of ice.[3]
Thermal hysteresis is easily measured in the lab with a nanolitre osmometer. Different organisms have different values of thermal hysteresis. The maximum level of thermal hysteresis shown by fish AFP is approximately -1.5°C (2.7°F). However, insect antifreeze proteins are 10–30 times more active than any known fish protein. This is probably because insects encounter lower temperatures on land than the –1°C or –2°C that fish face in freezing waters. During the extreme winter months, the spruce budworm can battle temperatures approaching –30°C and resist freezing,[2] though the Alaskan beetle Upis ceramboides can survive in a temperature of –60°C by using an antifreeze molecule that is not composed of proteins.[4]
The rate of cooling can influence the thermal hysteresis value of AFPs. Rapid cooling can substantially decrease the nonequilibrium freezing point, and hence the thermal hysteresis value. This means organisms may not be able to adapt to their subzero environment if the temperature drops abruptly.[2]
Freeze tolerance versus freeze avoidance
Species containing AFPs may be classified as:
Freeze avoidant: These species are able to prevent their body fluids from freezing altogether. Generally, the AFP function may be overcome at extremely cold temperatures, leading to rapid ice growth and death.
Freeze tolerant: These species are able to survive body fluid freezing. Some freeze tolerant species are thought to use AFPs as cryoprotectants to prevent the damages of freezing, but not freezing altogether. The exact mechanism is still unknown. However, it is thought AFPs may inhibit recrystallization and stabilize cell membranes to prevent damage by ice.[5] They may work in conjunction with protein ice nucleators (PINs) to control the rate of ice propagation following freezing.[5]
Diversity
There are many known nonhomologous types of AFP.
Fish AFPs

Antifreeze glycoproteins or AFGPs are found in Antarctic notothenioids and northern cod. They are 2.6-3.3 kD.[6]
Type I AFP is found in winter flounder, longhorn sculpin and shorthorn sculpin. It is the best documented AFP because it was the first to have its three dimensional structure determined.[7] Type I AFP consists of a single, long, amphipathic alpha helix, about 3.3-4.5 kD in size. There are three faces to the 3D structure: the hydrophobic, hydrophilic, and Thr-Asx face.[7]
Type I-hyp AFP (where hyp stands for hyperactive) are found in several righteye flounders. It is approximately 32 kD (two 17 kD dimeric molecules). The protein was isolated from the blood plasma of winter flounder. It is considerably better at depressing freezing temperature than most fish AFPs.[8]
Type II AFPs are found in sea raven, smelt and herring. They are cysteine-rich globular proteins containing five disulfide bonds.[9]
Type III AFPs are found in Antarctic eelpout. They exhibit similar overall hydrophobicity at ice binding surfaces to type I AFPs. They are approximately 6kD in size.[6]
Type IV AFPs are found in longhorn sculpins. They are alpha helical proteins rich in glutamate and glutamine.[10] This protein is approximately 12KDa in size and consists of a 4-helix bundle.[10] Its only posttranslational modification is a pyroglutamate residue, a cyclized glutamine residue at its N-terminal.[10] Scientists at the University of Guelph in Canada are currently examining the role of this pyroglutame residue in the antifreeze activity of type IV AFP from the longhorn sculpin.
Plant AFPs
The classification of AFPs became more complicated when antifreeze proteins from plant were discovered.[11] Plant AFPs are rather different from the other AFPs in the following aspects:
- They have much weaker thermal hysteresis activity when compared to other AFPs.[12]
- Their physiological function is likely in inhibiting the recrystallization of ice rather than in the preventing ice formation.[12]
- Most of them are evolved pathogenesis-related proteins, sometimes retaining antifungal properties.[12]
See also dehydrin
Insect AFPs
There are two types of insect antifreeze proteins, Tenebrio and Dendroides AFPs which are both in different insect families. They are similar to one another, both being hyperactive (i.e. greater thermal hysteresis value) and consist of varying numbers of 12- or 13-mer repeats of approximately 8.3 to 12.5 kD. Throughout the length of the protein, at least every sixth residue is a cysteine.[5]
Tenebrio or Type V AFPs are found in beetles,[13] whereas Dendroides or Choristoneura fumiferana AFPs are found in some Lepidoptera.
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
Sea ice organisms AFPs
AFPs were also found in microorganisms living in sea ice. The diatoms Fragilariopsis cylindrus and F. curta play a key role in polar sea ice communities, dominating the assemblages of both platelet layer and within pack ice. AFPs are widespread in these species, and the presence of AFP genes as a multigene family indicates the importance of this group for the genus Fragilariopsis.[16] AFPs identified in F. cylindrus belong to an AFP family which is represented in different taxa and can be found in other organisms related to sea ice (Colwellia spp., Navicula glaciei, Chaetoceros neogracile and Stephos longipes and Leucosporidium antarcticum[17][18]) and Antarctic inland ice bacteria (Flavobacteriaceae,[19][20] as well as in cold-tolerant fungi (Typhula ishikariensis, Lentinula edodes and Flammulina populicola.[21][22])
Evolution
The remarkable diversity and distribution of AFPs suggest the different types evolved recently in response to sea level glaciation occurring 1-2 million years ago in the Northern hemisphere and 10-30 million years ago in Antarctica. This independent development of similar adaptations is referred to as convergent evolution.[2] There are two reasons why many types of AFPs are able to carry out the same function despite their diversity:
- Although ice is uniformly composed of oxygen and hydrogen, it has many different surfaces exposed for binding. Different types of AFPs may interact with different surfaces.
- Although the five types of AFPs differ in their primary sequence of amino acids, when each folds into a functioning protein, they may share similarities in their three dimensional or tertiary structure that facilitates the same interactions with ice.[2][23]
Mechanisms of action
AFPs are thought to inhibit growth by an adsorption–inhibition mechanism.[24] They adsorb to nonbasal planes of ice, inhibiting thermodynamically favored ice growth.[25] The presence of a flat, rigid surface in some AFPs seems to facilitate its interaction with ice via Van der Waals force surface complementarity.[26]
Binding to ice
Normally, ice crystals grown in solution only exhibit the basal (0001) and prism faces (1010), and appear as round and flat discs.[3] However, it appears the presence of AFPs exposes other faces. It now appears the ice surface 2021 is the preferred binding surface, at least for AFP type I.[27] Through studies on type I AFP, ice and AFP were initially thought to interact through hydrogen bonding (Raymond and DeVries, 1977). However, when parts of the protein thought to facilitate this hydrogen bonding were mutated, the hypothesized decrease in antifreeze activity was not observed. Recent data suggest hydrophobic interactions could be the main contributor.[28] It is difficult to discern the exact mechanism of binding because of the complex water-ice interface. Currently, attempts to uncover the precise mechanism are being made through use of molecular modelling programs (molecular dynamics or the Monte Carlo method).[1][3]
Binding mechanism and antifreeze function
According to the structure and function study on the antifreeze protein from the fish winter flounder,[29] the antifreeze mechanism of the type-I AFP molecule was shown to be due to the binding to an ice nucleation structure in a zipper-like fashion through hydrogen bonding of the hydroxyl groups of its four Thr residues to the oxygens along the direction in ice lattice, subsequently stopping or retarding the growth of ice pyramidal planes so as to depress the freeze point.[29]
The above mechanism can be used to elucidate the structure-function relationship of other antifreeze proteins with the following two common features:
- recurrence of a Thr residue (or any other polar amino acid residue whose side-chain can form a hydrogen bond with water) in an 11-amino-acid period along the sequence concerned, and
- a high percentage of an Ala residue component therein.[29]
History
In the 1950s, Canadian scientist Scholander set out to explain how Arctic fish can survive in water colder than the freezing point of their blood. His experiments led him to believe there was “antifreeze” in the blood of Arctic fish.[1] Then in the late 1960s, animal biologist Arthur DeVries was able to isolate the antifreeze protein through his investigation of Antarctic fish.[30] These proteins were later called antifreeze glycoproteins (AFGPs) or antifreeze glycopeptides to distinguish them from newly discovered nonglycoprotein biological antifreeze agents (AFPs). DeVries worked with Robert Feeney (1970) to characterize the chemical and physical properties of antifreeze proteins.[31] In 1992, Griffith et al. documented their discovery of AFP in winter rye leaves. Around the same time, Urrutia, Duman and Knight (1992) documented thermal hysteresis protein in angiosperms. The next year, Duman and Olsen noted AFPs had also been discovered in over 23 species of angiosperms, including ones eaten by humans.[32] As well, they reported their presence in fungi and bacteria.
Name change
Recent attempts have been made to relabel antifreeze proteins as ice structuring proteins to more accurately represent their function and to dispose of any assumed negative relation between AFPs and automotive antifreeze, ethylene glycol. These two things are completely separate entities, and show loose similarity only in their function.[33]
Commercial applications
Commercially, there appear to be countless applications for antifreeze proteins.[34] Numerous fields would be able to benefit from the protection of tissue damage by freezing. Businesses are currently investigating the use of these proteins in:
- increasing freeze tolerance of crop plants and extending the harvest season in cooler climates
- improving farm fish production in cooler climates
- lengthening shelf life of frozen foods
- improving cryosurgery
- enhancing preservation of tissues for transplant or transfusion in medicine[35]
- therapy for hypothermia
Recent news
One recent, successful business endeavor has been the introduction of AFPs into ice cream and yogurt products. This ingredient, labelled ice-structuring protein, has been approved by the Food and Drug Administration. The proteins are isolated from fish and replicated, on a larger scale, in yeast.
There is concern from organizations opposed to genetically modified organisms (GMOs), arguing modified antifreeze proteins may cause inflammation.[36] However, as stated, ISPs have been approved for human consumption following diligent tests. Intake of AFPs in diet is likely substantial in most northerly and temperate regions already.[6] Given the known historic consumption of AFPs, it is safe to conclude their functional properties do not impart any toxicologic or allergenic effects in humans.[6]
As well, the transgenic process of ISP production is widely used in society already. This is how mass amounts of insulin are made to treat people with type I diabetes each year. The process does not impact the product; it merely makes production more efficient and prevents the death of many fish who would, otherwise, be killed for the extraction of such protein.
Currently, Unilever incorporates AFPs into some of its American products, including some popsicles and a new line of Breyers Light Double Churned ice cream bars. In ice cream, AFPs allow the production of very creamy, dense, reduced fat ice cream with fewer additives.[37] They control ice crystal growth brought on by thawing on the loading dock or kitchen table which drastically reduces texture quality.[38]
In November 2009, the Proceedings of the National Academy of Sciences published the discovery of a molecule in an Alaskan beetle that behaves like AFPs, but is composed of saccharides and fatty acids.[4]
A 2010 study demonstrated the stability of superheated water ice crystals in an AFP solution, showing while the proteins can inhibit freezing, they can also inhibit melting.[39]
References
- ^ a b c "Fishy Proteins". Pittsburgh Supercomputing Center. Retrieved 2011-08-24.
- ^ a b c d e f Fletcher GL, Hew CL, Davies PL (2001). "Antifreeze proteins of teleost fishes". Annu. Rev. Physiol. 63: 359–90. doi:10.1146/annurev.physiol.63.1.359. PMID 11181960.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Jorov A, Zhorov BS, Yang DS (2004). "Theoretical study of interaction of winter flounder antifreeze protein with ice". Protein Sci. 13 (6): 1524–37. doi:10.1110/ps.04641104. PMC 2279984. PMID 15152087.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Walters KR Jr, Serianni AS, Sformo T, Barnes BM, Duman JG (2009). "A nonprotein thermal hysteresis-producing xylomannan antifreeze in the freeze-tolerant Alaskan beetle Upis ceramboides". PNAS. Epub. ahead of print (48): 20210–5. doi:10.1073/pnas.0909872106. PMC 2787118. PMID 19934038.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Duman JG (2001). "Antifreeze and ice nucleator proteins in terrestrial arthropods". Annu. Rev. Physiol. 63: 327–57. doi:10.1146/annurev.physiol.63.1.327. PMID 11181959.
- ^ a b c d Crevel RW, Fedyk JK, Spurgeon MJ (2002). "Antifreeze proteins: characteristics, occurrence and human exposure". Food Chem. Toxicol. 40 (7): 899–903. PMID 12065210.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Duman JG, de Vries AL (1976). "Isolation, characterization, and physical properties of protein antifreezes from the winter flounder, Pseudopleuronectes americanus". Comp. Biochem. Physiol., B. 54 (3): 375–80. PMID 1277804.
- ^ Scotter AJ, Marshall CB, Graham LA, Gilbert JA, Garnham CP, Davies PL (2006). "The basis for hyperactivity of antifreeze proteins". Cryobiology. 53 (2): 229–39. doi:10.1016/j.cryobiol.2006.06.006. PMID 16887111.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ng NF, Hew CL (1992). "Structure of an antifreeze polypeptide from the sea raven. Disulfide bonds and similarity to lectin-binding proteins". J. Biol. Chem. 267 (23): 16069–75. PMID 1644794.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Deng G, Andrews DW, Laursen RA (1997). "Amino acid sequence of a new type of antifreeze protein, from the longhorn sculpin Myoxocephalus octodecimspinosis". FEBS Lett. 402 (1): 17–20. PMID 9013849.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Griffith M, Ala P, Yang DS, Hon WC, Moffatt BA (1992). "Antifreeze protein produced endogenously in winter rye leaves". Plant Physiol. 100 (2): 593–6. PMC 1075599. PMID 16653033.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Griffith M, Yaish MW (2004). "Antifreeze proteins in overwintering plants: a tale of two activities". Trends Plant Sci. 9 (8): 399–405. doi:10.1016/j.tplants.2004.06.007. PMID 15358271.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Graham LA, Liou YC, Walker VK, Davies PL (1997). "Hyperactive antifreeze protein from beetles". Nature. 388 (6644): 727–8. doi:10.1038/41908. PMID 9285581.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Daley ME, Spyracopoulos L, Jia Z, Davies PL, Sykes BD (2002). "Structure and dynamics of a beta-helical antifreeze protein". Biochemistry. 41 (17): 5515–25. doi:10.1021/bi0121252. PMID 11969412.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Leinala EK, Davies PL, Doucet D, Tyshenko MG, Walker VK, Jia Z (2002). "A beta-helical antifreeze protein isoform with increased activity. Structural and functional insights". J. Biol. Chem. 277 (36): 33349–52. doi:10.1074/jbc.M205575200. PMID 12105229.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Bayer-Giraldi M, Uhlig C, John U, Mock T, Valentin K (2010). "Antifreeze proteins in polar sea ice diatoms: diversity and gene expression in the genus Fragilariopsis". Environ. Microbiol. 12 (4): 1041–52. doi:10.1111/j.1462-2920.2009.02149.x. PMID 20105220.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Raymond JA, Fritsen C, Shen K (2007). "An ice-binding protein from an Antarctic sea ice bacterium". FEMS Microbiol. Ecol. 61 (2): 214–21. doi:10.1111/j.1574-6941.2007.00345.x. PMID 17651136.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kiko, R. (2010): Acquisition of freeze protection in a sea-ice crustacean through horizontal gene transfer? Polar Biology (33) 543-556.
- ^ Raymond JA, Christner BC, Schuster SC (2008). "A bacterial ice-binding protein from the Vostok ice core". Extremophiles. 12 (5): 713–7. doi:10.1007/s00792-008-0178-2. PMID 18622572.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ http://pnwfungi.org/pdf_files/manuscripts_volume_5/naf20105/naf2010514.pdf
- ^ Hoshino, T., Kiriaki, M., Ohgiya, S., Fujiwara, M., Kondo, H., Nishimiya, Y., et al. (2003) Antifreeze proteins from snow mold fungi. Can J Bot 81: 1175–1181.
- ^ Raymond JA, Janech MG (2009). "Ice-binding proteins from enoki and shiitake mushrooms". Cryobiology. 58 (2): 151–6. doi:10.1016/j.cryobiol.2008.11.009. PMID 19121299.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Chen L, DeVries AL, Cheng CH (1997). "Convergent evolution of antifreeze glycoproteins in Antarctic notothenioid fish and Arctic cod". Proc. Natl. Acad. Sci. U.S.A. 94 (8): 3817–22. PMC 20524. PMID 9108061.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Raymond JA, DeVries AL (1977). "Adsorption inhibition as a mechanism of freezing resistance in polar fishes". Proc. Natl. Acad. Sci. U.S.A. 74 (6): 2589–93. doi:10.1073/pnas.74.6.2589. PMC 432219. PMID 267952.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Raymond JA, Wilson P, DeVries AL (1989). "Inhibition of growth of nonbasal planes in ice by fish antifreezes". Proc. Natl. Acad. Sci. U.S.A. 86 (3): 881–5. doi:10.1073/pnas.86.3.881. PMC 286582. PMID 2915983.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Yang DS, Hon WC, Bubanko S, Xue Y, Seetharaman J, Hew CL, Sicheri F (1998). "Identification of the ice-binding surface on a type III antifreeze protein with a "flatness function" algorithm". Biophys. J. 74 (5): 2142–51. doi:10.1016/S0006-3495(98)77923-8. PMC 1299557. PMID 9591641.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Knight CA, Cheng CC, DeVries AL (1991). "Adsorption of alpha-helical antifreeze peptides on specific ice crystal surface planes". Biophys. J. 59 (2): 409–18. doi:10.1016/S0006-3495(91)82234-2. PMC 1281157. PMID 2009357.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Haymet AD, Ward LG, Harding MM, Knight CA (1998). "Valine substituted winter flounder 'antifreeze': preservation of ice growth hysteresis". FEBS Lett. 430 (3): 301–6. PMID 9688560.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Chou KC (1992). "Energy-optimized structure of antifreeze protein and its binding mechanism". J. Mol. Biol. 223 (2): 509–17. PMID 1738160.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ DeVries AL, Wohlschlag DE (1969). "Freezing resistance in some Antarctic fishes". Science. 163 (871): 1073–5. doi:10.1126/science.163.3871.1073. PMID 5764871.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ DeVries AL, Komatsu SK, Feeney RE (1970). "Chemical and physical properties of freezing point-depressing glycoproteins from Antarctic fishes". J. Biol. Chem. 245 (11): 2901–8. PMID 5488456.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Duman JG, Olsen TM (1993). "Thermal hysteresis protein activity in bacteria, fungi and phylogenetically diverse plants". Cryobiology. 30 (3): 322–328. doi:10.1006/cryo.1993.1031.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - ^ Clarke CJ, Buckley SL, Lindner N (2002). "Ice structuring proteins - a new name for antifreeze proteins". Cryo Letters. 23 (2): 89–92. PMID 12050776.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Antifreeze proteins and their genes: From basic research to business opportunity
- ^ Science Daily
- ^ Dortch, Eloise. (2006). Fishy GM yeast used to make ice-cream. Network of Concerned Farmers. Retrieved October 09, 2006
- ^ Creamy, Healthier Ice Cream? What’s the Catch?
- ^ Regand A, Goff HD (2006). "Ice recrystallization inhibition in ice cream as affected by ice structuring proteins from winter wheat grass". J. Dairy Sci. 89 (1): 49–57. doi:10.3168/jds.S0022-0302(06)72068-9. PMID 16357267.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Celik, Y; Graham, LA; Mok, YF; Bar, M; Davies, PL; Braslavsky, I (2010). "Superheating of Ice in the Presence of Ice Binding Proteins". Biophysical Journal. 98 (3): 245a. doi:10.1016/j.bpj.2009.12.1331.
{{cite journal}}: Unknown parameter|laysource=ignored (help); Unknown parameter|laysummary=ignored (help)
Further reading
- Haymett, A. (1999). "Winter Flounder 'anti-freeze' proteins: Synthesis and ice growth inhibition of analogues that probe the relative importance of hydrophobic and hydrogen bonding interactions". Journal of the American Chemical Society. 121 (5): 941–948. doi:10.1021/ja9801341. ISSN 0002-7863.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - Sicheri, F. (1995). "Ice-binding structure and mechanism of an antifreeze protein from winter flounder". Nature. 375 (6530): 427–431. doi:10.1038/375427a0. PMID 7760940.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)


![{\displaystyle [01{\overline {1}}2]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/25dd6fa8ecf7c3d8135f0b3bda61dde8032d89ce)