Clostridioides difficile: Difference between revisions
→References: moved |
→Genome: moved from Clostridium difficile diarrhea |
||
| Line 47: | Line 47: | ||
In 2012, scientists at University of Oxford sequenced ''C. difficile'' genomes from 486 cases arising over four years in Oxfordshire using next-generation sequencing technologies from Illumina.<ref name="pmid23259504">{{cite journal | author = Didelot X, Eyre DW, Cule M, Ip CL, Ansari MA, Griffiths D, Vaughan A, O'Connor L, Golubchik T, Batty EM, Piazza P, Wilson DJ, Bowden R, Donnelly PJ, Dingle KE, Wilcox M, Walker AS, Crook DW, A Peto TE, Harding RM | title = Microevolutionary analysis of Clostridium difficile genomes to investigate transmission | journal = Genome Biology | volume = 13 | issue = 12 | pages = R118 | date = December 2012 | pmid = 23259504 | doi = 10.1186/gb-2012-13-12-r118 | url = http://genomebiology.com/content/pdf/gb-2012-13-12-r118.pdf }}</ref> |
In 2012, scientists at University of Oxford sequenced ''C. difficile'' genomes from 486 cases arising over four years in Oxfordshire using next-generation sequencing technologies from Illumina.<ref name="pmid23259504">{{cite journal | author = Didelot X, Eyre DW, Cule M, Ip CL, Ansari MA, Griffiths D, Vaughan A, O'Connor L, Golubchik T, Batty EM, Piazza P, Wilson DJ, Bowden R, Donnelly PJ, Dingle KE, Wilcox M, Walker AS, Crook DW, A Peto TE, Harding RM | title = Microevolutionary analysis of Clostridium difficile genomes to investigate transmission | journal = Genome Biology | volume = 13 | issue = 12 | pages = R118 | date = December 2012 | pmid = 23259504 | doi = 10.1186/gb-2012-13-12-r118 | url = http://genomebiology.com/content/pdf/gb-2012-13-12-r118.pdf }}</ref> |
||
== Bacteriophage == |
|||
At least eight mainly temperate [[bacteriophage]]s have been isolated from ''C. difficile'', ranging in genome size from about 30 to about 60 kb.<ref name="Hargreaves">{{cite journal | author = Hargreaves KR, Clokie MR | title = ''Clostridium difficile'' phages: Still difficult? | journal = Frontiers in Microbiology | volume = 5 | pages = 184 | year = 2014 | pmid = 24808893 | pmc = 4009436 | doi = 10.3389/fmicb.2014.00184 }}</ref> Both environmentally and clinically derived ''C. difficile'' strains carry a diverse and prevalent set of [[prophage]]s.<ref name="Hargreaves"/> |
|||
==References== |
==References== |
||
Revision as of 05:24, 30 August 2014
| Clostridium difficile | |
|---|---|

| |
| C. difficile colonies on a blood agar plate | |
| |
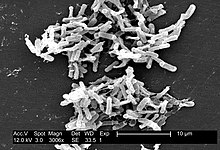
| Micrograph of Clostridium difficile | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | C. difficile
|
| Binomial name | |
| Clostridium difficile Hall & O'Toole, 1935
| |
Clostridia are motile bacteria, ubiquitous in nature and especially prevalent in soil. Under the microscope, they appear as long, irregular (often drumstick- or spindle-shaped) cells with a bulge at their terminal ends. Under Gram staining, C. difficile cells are Gram-positive and show optimum growth on blood agar at human body temperatures in the absence of oxygen. When stressed, the bacteria produce spores that can tolerate extreme conditions that the active bacteria cannot tolerate.[1]
C. difficile can become established in the human colon; it is present in 2–5% of the adult population.[1]

Pathogenic C. difficile strains produce multiple toxins. The most well-characterized are enterotoxin (Clostridium difficile toxin A) and cytotoxin (Clostridium difficile toxin B), both of which can produce diarrhea and inflammation in infected patients, although their relative contributions have been debated.[1] Toxins A and B are glucosyltransferases that target and inactivate the Rho family of GTPases. Toxin B (cytotoxin) induces actin depolymerization by a mechanism correlated with a decrease in the ADP-ribosylation of the low molecular mass GTP-binding Rho proteins.[2] Another toxin, binary toxin, has also been described, but its role in disease is not fully understood.[3]
Antibiotic treatment of CDIs can be difficult, due both to antibiotic resistance and physiological factors of the bacteria (spore formation, protective effects of the pseudomembrane).[1] The emergence of a new, highly toxic strain of C. difficile, resistant to fluoroquinolone antibiotics, such as ciprofloxacin and levofloxacin, said to be causing geographically dispersed outbreaks in North America was reported in 2005.[4] The U.S. Centers for Disease Control in Atlanta warned of the emergence of an epidemic strain with increased virulence, antibiotic resistance, or both.[5]
C. difficile is transmitted from person to person by the fecal-oral route. However, the organism forms heat-resistant spores that are not killed by alcohol-based hand cleansers or routine surface cleaning. Thus, these spores survive in clinical environments for long periods. Because of this, the bacteria can be cultured from almost any surface. Once spores are ingested, their acid-resistance allows them to pass through the stomach unscathed. They germinate and multiply into vegetative cells in the colon upon exposure to bile acids.
In 2005, molecular analysis led to the identification of the C. difficile strain type characterized as group BI by restriction endonuclease analysis , as North American pulse-field-type NAP1 by pulsed-field gel electrophoresis and as ribotype 027; the differing terminology reflects the predominant techniques used for epidemiological typing. This strain is referred to as C. difficile BI/NAP1/027.[6]
Genome
| NCBI genome ID | 535? |
|---|---|
| Ploidy | haploid |
| Genome size | 4.3 Mb |
| Number of chromosomes | 1 |
| Year of completion | 2005 |
The first complete genome sequence of a C. difficile strain was first published in 2005 by Sanger Institute in the UK. This was of the strain 630, a virulent and multiple drug-resistant strain isolated in Switzerland in 1982. Scientists at Sanger Institute have sequenced genomes of about 30 C. difficile isolates using next-generation sequencing technologies from 454 Life Sciences and Illumina.[7]
Researchers at McGill University in Montreal sequenced the genome of the highly virulent Quebec strain of C. difficile in 2005 using ultra-high-throughput sequencing technology. The tests involved doing 400,000 DNA parallel-sequencing reactions of the bacterium's genome, which had been fragmented for sequencing. These sequences were assembled computationally to form a complete genome sequence.[4][8]
In 2012, scientists at University of Oxford sequenced C. difficile genomes from 486 cases arising over four years in Oxfordshire using next-generation sequencing technologies from Illumina.[9]
Bacteriophage
At least eight mainly temperate bacteriophages have been isolated from C. difficile, ranging in genome size from about 30 to about 60 kb.[10] Both environmentally and clinically derived C. difficile strains carry a diverse and prevalent set of prophages.[10]
References
- ^ a b c d Cite error: The named reference
Sherriswas invoked but never defined (see the help page). - ^ Just I, Selzer J, von Eichel-Streiber C, Aktories K (1995). "The low molecular mass GTP-binding protein Rh is affected by toxin a from Clostridium difficile". The Journal of Clinical Investigation. 95 (3): 1026–31. doi:10.1172/JCI117747. PMC 441436. PMID 7883950.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Barth H, Aktories K, Popoff MR, Stiles BG (2004). "Binary Bacterial Toxins: Biochemistry, Biology, and Applications of Common Clostridium and Bacillus Proteins". Microbiology and Molecular Biology Reviews : MMBR. 68 (3): 373–402, table of contents. doi:10.1128/MMBR.68.3.373-402.2004. PMC 515256. PMID 15353562.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, René P, Monczak Y, Dascal A (2005 month = December). "A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality". The New England Journal of Medicine. 353 (23): 2442–9. doi:10.1056/NEJMoa051639. PMID 16322602.
{{cite journal}}: Check date values in:|year=(help)CS1 maint: multiple names: authors list (link) - ^ McDonald LC (August 2005). "Clostridium difficile: responding to a new threat from an old enemy" (PDF). Infection Control and Hospital Epidemiology : The Official Journal of the Society of Hospital Epidemiologists of America. 26 (8): 672–5. doi:10.1086/502600. PMID 16156321.
- ^ Rupnik M, Wilcox MH, Gerding DN (July 2009). "Clostridium difficile infection: New developments in epidemiology and pathogenesis". Nature Reviews. Microbiology. 7 (7): 526–36. doi:10.1038/nrmicro2164. PMID 19528959.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ He M, Sebaihia M, Lawley TD, Stabler RA, Dawson LF, Martin MJ, Holt KE, Seth-Smith HM, Quail MA, Rance R, Brooks K, Churcher C, Harris D, Bentley SD, Burrows C, Clark L, Corton C, Murray V, Rose G, Thurston S, van Tonder A, Walker D, Wren BW, Dougan G, Parkhill J (April 2010). "Evolutionary dynamics of Clostridium difficile over short and long time scales" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 107 (16): 7527–32. doi:10.1073/pnas.0914322107. PMC 2867753. PMID 20368420.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Scientists map C. difficile strain - Institute of Public Affairs, Montreal
- ^ Didelot X, Eyre DW, Cule M, Ip CL, Ansari MA, Griffiths D, Vaughan A, O'Connor L, Golubchik T, Batty EM, Piazza P, Wilson DJ, Bowden R, Donnelly PJ, Dingle KE, Wilcox M, Walker AS, Crook DW, A Peto TE, Harding RM (December 2012). "Microevolutionary analysis of Clostridium difficile genomes to investigate transmission" (PDF). Genome Biology. 13 (12): R118. doi:10.1186/gb-2012-13-12-r118. PMID 23259504.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Hargreaves KR, Clokie MR (2014). "Clostridium difficile phages: Still difficult?". Frontiers in Microbiology. 5: 184. doi:10.3389/fmicb.2014.00184. PMC 4009436. PMID 24808893.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
