Chemical cycling: Difference between revisions
Wavelength (talk | contribs) |
No edit summary |
||
| Line 10: | Line 10: | ||
== Venus == |
== Venus == |
||
The majority of known chemical cycles on Venus involve its dense atmosphere and compounds of carbon and sulphur, the most significant being a strong carbon dioxide cycle.<ref name="MillsAllen2007">{{cite journal|last1=Mills|first1=Franklin P.|last2=Allen|first2=Mark|title=A review of selected issues concerning the chemistry in Venus’ middle atmosphere|journal=Planetary and Space Science|volume=55|issue=12|year=2007|pages=1729–1740|issn=00320633|doi=10.1016/j.pss.2007.01.012}}</ref> The lack of a complete carbon cycle including a geochemical carbon cycle, for example, is thought to be a cause of its [[runaway greenhouse effect]], due to the lack of a substantial carbon sink.<ref>{{cite web |url=http://home.case.edu/~sjr16/venus.html |title=Venus |author=Nick Strobel |accessdate=17 February 2009 }}</ref> |
The majority of known chemical cycles on [[Venus]] involve its dense atmosphere and compounds of carbon and sulphur, the most significant being a strong carbon dioxide cycle.<ref name="MillsAllen2007">{{cite journal|last1=Mills|first1=Franklin P.|last2=Allen|first2=Mark|title=A review of selected issues concerning the chemistry in Venus’ middle atmosphere|journal=Planetary and Space Science|volume=55|issue=12|year=2007|pages=1729–1740|issn=00320633|doi=10.1016/j.pss.2007.01.012}}</ref> The lack of a complete carbon cycle including a geochemical carbon cycle, for example, is thought to be a cause of its [[runaway greenhouse effect]], due to the lack of a substantial carbon sink.<ref>{{cite web |url=http://home.case.edu/~sjr16/venus.html |title=Venus |author=Nick Strobel |accessdate=17 February 2009 }}</ref> Sulphur cycles including sulphur oxide cycles also occur, sulphur oxide in the upper atmosphere and results in the presence of [[Sulfuric acid]]<ref name="JessupMarcq2015">{{cite journal|last1=Jessup|first1=Kandis Lea|last2=Marcq|first2=Emmanuel|last3=Mills|first3=Franklin|last4=Mahieux|first4=Arnaud|last5=Limaye|first5=Sanjay|last6=Wilson|first6=Colin|last7=Allen|first7=Mark|last8=Bertaux|first8=Jean-Loup|last9=Markiewicz|first9=Wojciech|last10=Roman|first10=Tony|last11=Vandaele|first11=Ann-Carine|last12=Wilquet|first12=Valerie|last13=Yung|first13=Yuk|title=Coordinated Hubble Space Telescope and Venus Express Observations of Venus’ upper cloud deck|journal=Icarus|volume=258|year=2015|pages=309–336|issn=00191035|doi=10.1016/j.icarus.2015.05.027}}</ref> in turn returns to oxides through photolysis.<ref name="ZhangLiang2010">{{cite journal|last1=Zhang|first1=Xi|last2=Liang|first2=Mao-Chang|last3=Montmessin|first3=Franck|last4=Bertaux|first4=Jean-Loup|last5=Parkinson|first5=Christopher|last6=Yung|first6=Yuk L.|title=Photolysis of sulphuric acid as the source of sulphur oxides in the mesosphere of Venus|journal=Nature Geoscience|volume=3|issue=12|year=2010|pages=834–837|issn=1752-0894|doi=10.1038/ngeo989}}</ref> Indications also suggest an ozone cycle on Venus similar to that of Earth's.<ref name="MontmessinBertaux2011">{{cite journal|last1=Montmessin|first1=F.|last2=Bertaux|first2=J.-L.|last3=Lefèvre|first3=F.|last4=Marcq|first4=E.|last5=Belyaev|first5=D.|last6=Gérard|first6=J.-C.|last7=Korablev|first7=O.|last8=Fedorova|first8=A.|last9=Sarago|first9=V.|last10=Vandaele|first10=A.C.|title=A layer of ozone detected in the nightside upper atmosphere of Venus|journal=Icarus|volume=216|issue=1|year=2011|pages=82–85|issn=00191035|doi=10.1016/j.icarus.2011.08.010}}</ref> |
||
== Earth == |
== Earth == |
||
[[Image:Water cycle.png|thumb|320px|right|Earth's [[water cycle]].]] |
[[Image:Water cycle.png|thumb|320px|right|Earth's [[water cycle]].]] |
||
A number of different types of chemical cycles geochemical cycles occur on Earth. Biogeochemical cycles play an important role in sustaining the biosphere. |
A number of different types of chemical cycles geochemical cycles occur on Earth. Biogeochemical cycles play an important role in sustaining the biosphere. |
||
Notable chemical cycles on Earth include: |
Notable active chemical cycles on Earth include: |
||
* [[Carbon cycle]] - consisting of an [[atmospheric carbon cycle]], [[terrestrial biological carbon cycle]], [[oceanic carbon cycle]] and [[Carbon sink|geological carbon cycle]] |
|||
* [[Carbon cycle]] |
|||
* [[Nitrogen cycle]] - which converts nitrogen between its forms through [[nitrogen fixation|fixation]], [[ammonification]], [[nitrification]], and [[denitrification]] |
|||
* [[Nitrogen cycle]] |
|||
* [[Oxygen cycle]] and [[Ozone–oxygen cycle]] |
* [[Oxygen cycle]] and [[Ozone–oxygen cycle]] - a [[biogeochemical cycle]] of circulating oxygen between the atmosphere, [[biosphere]] (the global sum of all ecosystems), and the lithosphere |
||
* [[Ozone-oxygen cycle]] - continually regenerates ozone in the atmosphere and converts [[Ultraviolet|ultraviolet radiation]] (UV) into [[heat]] |
|||
* [[Water cycle]] |
|||
* [[Water cycle]] - moves water continuously on, above and below the surface shifting between states of liquid, solution, ice and vapour |
|||
* [[Hydrogen cycle]] |
|||
* [[Methane cycle]] - moves methane between geological and biogeochemical sources and reactions in the atmosphere |
|||
* [[Phosphorus cycle]] |
|||
* [[Hydrogen cycle]] - a biogeochemical cycle brought about by a combination of biological and abiological processes |
|||
* [[Sulfur cycle]] |
|||
* [[Phosphorus cycle]] - the movement of phosphorus through the lithosphere, hydrosphere, and biosphere |
|||
* [[Rock cycle]] |
|||
* [[Sulfur cycle]] - a biogeochemical process resulting form the mineralization of organic sulfur, oxidation, reduction and incorporation into organic compounds |
|||
* [[Mercury cycle]] |
|||
* [[Carbonate–silicate cycle]] transforms [[silicate]] rocks to [[Carbonate rock|carbonate]] rocks by [[weathering]] and [[sedimentation]] and transforms carbonate rocks back into silicates by [[metamorphism]] and [[magmatism]].<ref>{{Cite journal|url = http://shadow.eas.gatech.edu/~jean/paleo/Berner_1983.pdf|title = The Carbonate-Silicate Geochemical Cycle and its Effect on Atmospheric Carbon Dioxide over the Past 100 Million Years|last = Berner|first = Robert|date = September 1983|journal = American Journal of Science|doi = |pmid = |access-date = Feb 3, 2015|first2 = Antonio|last2 = Lasaga|volume = 283|pages = 641–683|last3 = Garrels|first3 = Robert}}</ref> |
|||
* [[Rock cycle]] - switches rock between its three forms: sedimentary, metamorphic, and igneous |
|||
* [[Mercury cycle]] - a biogeochemical process in which naturally occurring mercury is bioaccumulated before recombining with sulfur and returning to geological sources as sediments |
|||
Other chemical cycles include [[hydrogen peroxide]].<ref name="AllenGonzález Abad2013">{{cite journal|last1=Allen|first1=Nicholas D.C.|last2=González Abad|first2=Gonzalo|last3=Bernath|first3=Peter F.|last4=Boone|first4=Chris D.|title=Satellite observations of the global distribution of hydrogen peroxide (H2O2) from ACE|journal=Journal of Quantitative Spectroscopy and Radiative Transfer|volume=115|year=2013|pages=66–77|issn=00224073|doi=10.1016/j.jqsrt.2012.09.008}}</ref> |
|||
== Mars == |
== Mars == |
||
[[File:PIA19088-MarsCuriosityRover-MethaneSource-20141216.png|thumb|[[Atmosphere of Mars#Methane|Methane]] |
[[File:PIA19088-MarsCuriosityRover-MethaneSource-20141216.png|thumb|Possible sources of a hypothesized [[Atmosphere of Mars#Methane|Martian Methane]] cycle.]] |
||
Recent evidence suggests that similar chemical cycles to Earth's occur on a lesser scale on Mars, facilitated by the thin atmosphere, including possible carbon,<ref name="EdwardsEhlmann2015">{{cite journal|last1=Edwards|first1=Christopher S.|last2=Ehlmann|first2=Bethany L.|title=Carbon sequestration on Mars|journal=Geology|volume=43|issue=10|year=2015|pages=863–866|issn=0091-7613|doi=10.1130/G36983.1}}</ref> water,<ref name="Machtoub2012">{{cite journal|last1=Machtoub|first1=G.|title=Modeling the hydrological cycle on Mars|journal=Journal of Advances in Modeling Earth Systems|volume=4|year=2012|issn=1942-2466|doi=10.1029/2011MS000069}}</ref> methane,<ref name="WrayEhlmann2011">{{cite journal|last1=Wray|first1=James J.|last2=Ehlmann|first2=Bethany L.|title=Geology of possible Martian methane source regions|journal=Planetary and Space Science|volume=59|issue=2-3|year=2011|pages=196–202|issn=00320633|doi=10.1016/j.pss.2010.05.006}}</ref> oxygen,<ref name="FarquharThiemens2000">{{cite journal|last1=Farquhar|first1=James|last2=Thiemens|first2=Mark H.|title=Oxygen cycle of the Martian atmosphere-regolith system: Δ17O of secondary phases in Nakhla and Lafayette|journal=Journal of Geophysical Research: Planets|volume=105|issue=E5|year=2000|pages=11991–11997|issn=01480227|doi=10.1029/1999JE001194}}</ref> ozone,<ref name="MontmessinLefèvre2013">{{cite journal|last1=Montmessin|first1=Franck|last2=Lefèvre|first2=Franck|title=Transport-driven formation of a polar ozone layer on Mars|journal=Nature Geoscience|volume=6|issue=11|year=2013|pages=930–933|issn=1752-0894|doi=10.1038/ngeo1957}}</ref> and nitrogen<ref name="BoxeHand2012">{{cite journal|last1=Boxe|first1=C.S.|last2=Hand|first2=K.P.|last3=Nealson|first3=K.H.|last4=Yung|first4=Y.L.|last5=Saiz-Lopez|first5=A.|title=An active nitrogen cycle on Mars sufficient to support a subsurface biosphere|journal=International Journal of Astrobiology|volume=11|issue=02|year=2012|pages=109–115|issn=1473-5504|doi=10.1017/S1473550411000401}}</ref> cycles. Many studies point to significantly more active chemical cycles on Mars in the past. |
Recent evidence suggests that similar chemical cycles to Earth's occur on a lesser scale on [[Mars]], facilitated by the thin atmosphere, including possible carbon,<ref name="EdwardsEhlmann2015">{{cite journal|last1=Edwards|first1=Christopher S.|last2=Ehlmann|first2=Bethany L.|title=Carbon sequestration on Mars|journal=Geology|volume=43|issue=10|year=2015|pages=863–866|issn=0091-7613|doi=10.1130/G36983.1}}</ref> water,<ref name="Machtoub2012">{{cite journal|last1=Machtoub|first1=G.|title=Modeling the hydrological cycle on Mars|journal=Journal of Advances in Modeling Earth Systems|volume=4|year=2012|issn=1942-2466|doi=10.1029/2011MS000069}}</ref> sulphur,<ref name="KingMcLennan2010">{{cite journal|last1=King|first1=P. L.|last2=McLennan|first2=S. M.|title=Sulfur on Mars|journal=Elements|volume=6|issue=2|year=2010|pages=107–112|issn=1811-5209|doi=10.2113/gselements.6.2.107}}</ref> methane,<ref name="WrayEhlmann2011">{{cite journal|last1=Wray|first1=James J.|last2=Ehlmann|first2=Bethany L.|title=Geology of possible Martian methane source regions|journal=Planetary and Space Science|volume=59|issue=2-3|year=2011|pages=196–202|issn=00320633|doi=10.1016/j.pss.2010.05.006}}</ref> oxygen,<ref name="FarquharThiemens2000">{{cite journal|last1=Farquhar|first1=James|last2=Thiemens|first2=Mark H.|title=Oxygen cycle of the Martian atmosphere-regolith system: Δ17O of secondary phases in Nakhla and Lafayette|journal=Journal of Geophysical Research: Planets|volume=105|issue=E5|year=2000|pages=11991–11997|issn=01480227|doi=10.1029/1999JE001194}}</ref> ozone,<ref name="MontmessinLefèvre2013">{{cite journal|last1=Montmessin|first1=Franck|last2=Lefèvre|first2=Franck|title=Transport-driven formation of a polar ozone layer on Mars|journal=Nature Geoscience|volume=6|issue=11|year=2013|pages=930–933|issn=1752-0894|doi=10.1038/ngeo1957}}</ref> and nitrogen<ref name="BoxeHand2012">{{cite journal|last1=Boxe|first1=C.S.|last2=Hand|first2=K.P.|last3=Nealson|first3=K.H.|last4=Yung|first4=Y.L.|last5=Saiz-Lopez|first5=A.|title=An active nitrogen cycle on Mars sufficient to support a subsurface biosphere|journal=International Journal of Astrobiology|volume=11|issue=02|year=2012|pages=109–115|issn=1473-5504|doi=10.1017/S1473550411000401}}</ref> cycles. Many studies point to significantly more active chemical cycles on Mars in the past, however the [[faint young Sun paradox]] has proved problematic in determining chemical cycles involved in early climate models of the planet.<ref name="WordsworthForget2013">{{cite journal|last1=Wordsworth|first1=R.|last2=Forget|first2=F.|last3=Millour|first3=E.|last4=Head|first4=J.W.|last5=Madeleine|first5=J.-B.|last6=Charnay|first6=B.|title=Global modelling of the early martian climate under a denser CO2 atmosphere: Water cycle and ice evolution|journal=Icarus|volume=222|issue=1|year=2013|pages=1–19|issn=00191035|doi=10.1016/j.icarus.2012.09.036}}</ref> |
||
== Jupiter == |
== Jupiter == |
||
[[Image:PIA04433 Jupiter Torus Diagram.jpg|thumb|right|Jupiter's gas toruses generated by [[Io (moon)|Io]] (green) and [[Europa (moon)|Europa]] (blue)]] |
[[Image:PIA04433 Jupiter Torus Diagram.jpg|thumb|right|Jupiter's gas toruses generated by [[Io (moon)|Io]] (green) and [[Europa (moon)|Europa]] (blue)]] |
||
Recent studies indicate water-ammonia (hyrdological) and [[hydrogen sulfide]] cycles on Jupiter.<ref name="PalotaiDowling2014">{{cite journal|last1=Palotai|first1=Csaba|last2=Dowling|first2=Timothy E.|last3=Fletcher|first3=Leigh N.|title=3D Modeling of interactions between Jupiter’s ammonia clouds and large anticyclones|journal=Icarus|volume=232|year=2014|pages=141–156|issn=00191035|doi=10.1016/j.icarus.2014.01.005}}</ref> |
Recent studies indicate water-ammonia (hyrdological) and [[hydrogen sulfide]] cycles on [[Jupiter]].<ref name="PalotaiDowling2014">{{cite journal|last1=Palotai|first1=Csaba|last2=Dowling|first2=Timothy E.|last3=Fletcher|first3=Leigh N.|title=3D Modeling of interactions between Jupiter’s ammonia clouds and large anticyclones|journal=Icarus|volume=232|year=2014|pages=141–156|issn=00191035|doi=10.1016/j.icarus.2014.01.005}}</ref> |
||
Significant chemical cycles exist on Jupiter's moons. Recent evidence points to |
Significant chemical cycles exist on Jupiter's moons. Recent evidence points to [[Europa (moon)|Europa]] possessing several active cycles, most notably a water cycle.<ref name="KattenhornProckter2014">{{cite journal|last1=Kattenhorn|first1=Simon A.|last2=Prockter|first2=Louise M.|title=Evidence for subduction in the ice shell of Europa|journal=Nature Geoscience|volume=7|issue=10|year=2014|pages=762–767|issn=1752-0894|doi=10.1038/ngeo2245}}</ref> Other studies suggest an oxygen and carbon dioxide cycle.<ref name="HandChyba2006">{{cite journal|last1=Hand|first1=Kevin P.|last2=Chyba|first2=Christopher F.|last3=Carlson|first3=Robert W.|last4=Cooper|first4=John F.|title=Clathrate Hydrates of Oxidants in the Ice Shell of Europa|journal=Astrobiology|volume=6|issue=3|year=2006|pages=463–482|issn=1531-1074|doi=10.1089/ast.2006.6.463}}</ref> Io and Europa, appear to have sulphur cycles involving their lithospheres.<ref name="Battaglia2014">{{cite journal |title=Io's theothermal (sulfur) - Lithosphere cycle inferred from sulfur solubility modeling of Pele's magma supply |journal=Icarus |first1=Steven M. |last1=Battaglia |first2=Michael A. |last2=Stewart |first3=Susan W. |last3=Kieffer |volume=235 |pages=123–129 |date=June 2014 |doi=10.1016/j.icarus.2014.03.019 |bibcode=2014Icar.235.123B}}</ref> In addition, the [[Io plasma torus]] contributes to a sulphur cycle on Jupiter and Ganymede.<ref name="Cheng1984">{{cite journal|last1=Cheng|first1=Andrew F.|title=Escape of sulfur and oxygen from Io|journal=Journal of Geophysical Research|volume=89|issue=A6|year=1984|pages=3939|issn=0148-0227|doi=10.1029/JA089iA06p03939}}</ref> Studies also imply active oxygen cycles on [[Ganymede]] and [[Callisto]].<ref>Vidal RA, Bahr D, Baragiola RA, Peters M. Oxygen on Ganymede: laboratory studies. Science. 1997;276(5320):1839-42.</ref> |
||
== Saturn == |
== Saturn == |
||
| Line 41: | Line 46: | ||
Some studies suggest an ammonia cycle similar to Jupiter's also operates on Saturn in addition to chemical cycles induced by photolysis.<ref name="WestBaines2009">{{cite journal|last1=West|first1=R. A.|last2=Baines|first2=K. H.|last3=Karkoschka|first3=E.|last4=Sánchez-Lavega|first4=A.|title=Clouds and Aerosols in Saturn's Atmosphere|year=2009|pages=161–179|doi=10.1007/978-1-4020-9217-6_7}}</ref> |
Some studies suggest an ammonia cycle similar to Jupiter's also operates on Saturn in addition to chemical cycles induced by photolysis.<ref name="WestBaines2009">{{cite journal|last1=West|first1=R. A.|last2=Baines|first2=K. H.|last3=Karkoschka|first3=E.|last4=Sánchez-Lavega|first4=A.|title=Clouds and Aerosols in Saturn's Atmosphere|year=2009|pages=161–179|doi=10.1007/978-1-4020-9217-6_7}}</ref> |
||
The cycles of its moons are of particular interest. Observations by [[Cassini–Huygens]] of [[Titan (moon)|Titan]]'s atmosphere and interactions with its liquid mantle give rise to has several active chemical cycles including a methane,<ref name="AtreyaAdams2006">{{cite journal|last1=Atreya|first1=Sushil K.|last2=Adams|first2=Elena Y.|last3=Niemann|first3=Hasso B.|last4=Demick-Montelara|first4=Jaime E.|last5=Owen|first5=Tobias C.|last6=Fulchignoni|first6=Marcello|last7=Ferri|first7=Francesca|last8=Wilson|first8=Eric H.|title=Titan's methane cycle|journal=Planetary and Space Science|volume=54|issue=12|year=2006|pages=1177–1187|issn=00320633|doi=10.1016/j.pss.2006.05.028}}</ref> hydrocarbon,<ref name="TobieChoukroun2009">{{cite journal|last1=Tobie|first1=G|last2=Choukroun|first2=M|last3=Grasset|first3=O|last4=Le Mouelic|first4=S|last5=Lunine|first5=J.I|last6=Sotin|first6=C|last7=Bourgeois|first7=O|last8=Gautier|first8=D|last9=Hirtzig|first9=M|last10=Lebonnois|first10=S|last11=Le Corre|first11=L|title=Evolution of Titan and implications for its hydrocarbon cycle|journal=Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences|volume=367|issue=1889|year=2009|pages=617–631|issn=1364-503X|doi=10.1098/rsta.2008.0246}}</ref> hydrogen,<ref name="LebonnoisBakes2003">{{cite journal|last1=Lebonnois|first1=S.ébastien|last2=Bakes|first2=E.L.O.|last3=McKay|first3=Christopher P.|title=Atomic and molecular hydrogen budget in Titan’s atmosphere|journal=Icarus|volume=161|issue=2|year=2003|pages=474–485|issn=00191035|doi=10.1016/S0019-1035(02)00039-8}}</ref> and carbon<ref name="ChoukrounSotin2012">{{cite journal|last1=Choukroun|first1=M.|last2=Sotin|first2=C.|title=Is Titan's shape caused by its meteorology and carbon cycle?|journal=Geophysical Research Letters|volume=39|issue=4|year=2012|pages=n/a–n/a|issn=00948276|doi=10.1029/2011GL050747}}</ref> cycles. Enceladus has an active hydrological, silicate and possibly a nitrogen cycle. |
The cycles of its moons are of particular interest. Observations by [[Cassini–Huygens]] of [[Titan (moon)|Titan]]'s atmosphere and interactions with its liquid mantle give rise to has several active chemical cycles including a methane,<ref name="AtreyaAdams2006">{{cite journal|last1=Atreya|first1=Sushil K.|last2=Adams|first2=Elena Y.|last3=Niemann|first3=Hasso B.|last4=Demick-Montelara|first4=Jaime E.|last5=Owen|first5=Tobias C.|last6=Fulchignoni|first6=Marcello|last7=Ferri|first7=Francesca|last8=Wilson|first8=Eric H.|title=Titan's methane cycle|journal=Planetary and Space Science|volume=54|issue=12|year=2006|pages=1177–1187|issn=00320633|doi=10.1016/j.pss.2006.05.028}}</ref> hydrocarbon,<ref name="TobieChoukroun2009">{{cite journal|last1=Tobie|first1=G|last2=Choukroun|first2=M|last3=Grasset|first3=O|last4=Le Mouelic|first4=S|last5=Lunine|first5=J.I|last6=Sotin|first6=C|last7=Bourgeois|first7=O|last8=Gautier|first8=D|last9=Hirtzig|first9=M|last10=Lebonnois|first10=S|last11=Le Corre|first11=L|title=Evolution of Titan and implications for its hydrocarbon cycle|journal=Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences|volume=367|issue=1889|year=2009|pages=617–631|issn=1364-503X|doi=10.1098/rsta.2008.0246}}</ref> hydrogen,<ref name="LebonnoisBakes2003">{{cite journal|last1=Lebonnois|first1=S.ébastien|last2=Bakes|first2=E.L.O.|last3=McKay|first3=Christopher P.|title=Atomic and molecular hydrogen budget in Titan’s atmosphere|journal=Icarus|volume=161|issue=2|year=2003|pages=474–485|issn=00191035|doi=10.1016/S0019-1035(02)00039-8}}</ref> and carbon<ref name="ChoukrounSotin2012">{{cite journal|last1=Choukroun|first1=M.|last2=Sotin|first2=C.|title=Is Titan's shape caused by its meteorology and carbon cycle?|journal=Geophysical Research Letters|volume=39|issue=4|year=2012|pages=n/a–n/a|issn=00948276|doi=10.1029/2011GL050747}}</ref> cycles. Enceladus has an active hydrological, silicate and possibly a nitrogen cycle.<ref name="ParkinsonLiang2007">{{cite journal|last1=Parkinson|first1=C. D.|last2=Liang|first2=M.-C.|last3=Hartman|first3=H.|last4=Hansen|first4=C. J.|last5=Tinetti|first5=G.|last6=Meadows|first6=V.|last7=Kirschvink|first7=J. L.|last8=Yung|first8=Y. L.|title=Enceladus: Cassini observations and implications for the search for life|journal=Astronomy and Astrophysics|volume=463|issue=1|year=2007|pages=353–357|issn=0004-6361|doi=10.1051/0004-6361:20065773}}</ref><ref name="ParkinsonLiang2008">{{cite journal|last1=Parkinson|first1=Christopher D.|last2=Liang|first2=Mao-Chang|last3=Yung|first3=Yuk L.|last4=Kirschivnk|first4=Joseph L.|title=Habitability of Enceladus: Planetary Conditions for Life|journal=Origins of Life and Evolution of Biospheres|volume=38|issue=4|year=2008|pages=355–369|issn=0169-6149|doi=10.1007/s11084-008-9135-4}}</ref> |
||
== Uranus == |
== Uranus == |
||
Revision as of 13:51, 12 March 2016

Chemical cycling describes systems of repeated circulation of chemicals between other compounds, states and materials, and back to their original state, that occurs in space, and on many objects in space including the Earth. Active chemical cycling is known to occur in stars, many planets and natural satellites.
Chemical cycling plays a large role in sustaining planetary atmospheres, liquids and biological processes and can greatly influence weather and climate. Some chemical cycles release renewable energy, others may give rise to complex chemical reactions, organic compounds and prebiotic chemistry. On terrestrial bodies such as the Earth, chemical cycles involving the lithosphere are known as geochemical cycles. Ongoing geochemical cycles are one of the main attributes of geologically active worlds. A chemical cycle involving a biosphere is known as a biogeochemical cycle.
The Sun, other stars and star systems
In most hydrogen fusing stars, including the Sun, a chemical cycle involved in stellar nucleosynthesis occurs which is known as a carbon-nitrogen-oxygen or (CNO cycle). In addition to this cycle, stars also have a helium cycle.[1] Various cycles involving gas and dust have been found to occur in galaxies.[2]
Venus
The majority of known chemical cycles on Venus involve its dense atmosphere and compounds of carbon and sulphur, the most significant being a strong carbon dioxide cycle.[3] The lack of a complete carbon cycle including a geochemical carbon cycle, for example, is thought to be a cause of its runaway greenhouse effect, due to the lack of a substantial carbon sink.[4] Sulphur cycles including sulphur oxide cycles also occur, sulphur oxide in the upper atmosphere and results in the presence of Sulfuric acid[5] in turn returns to oxides through photolysis.[6] Indications also suggest an ozone cycle on Venus similar to that of Earth's.[7]
Earth
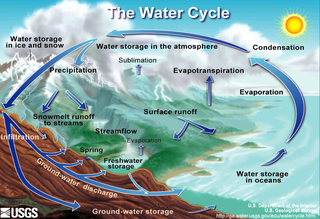
A number of different types of chemical cycles geochemical cycles occur on Earth. Biogeochemical cycles play an important role in sustaining the biosphere. Notable active chemical cycles on Earth include:
- Carbon cycle - consisting of an atmospheric carbon cycle, terrestrial biological carbon cycle, oceanic carbon cycle and geological carbon cycle
- Nitrogen cycle - which converts nitrogen between its forms through fixation, ammonification, nitrification, and denitrification
- Oxygen cycle and Ozone–oxygen cycle - a biogeochemical cycle of circulating oxygen between the atmosphere, biosphere (the global sum of all ecosystems), and the lithosphere
- Ozone-oxygen cycle - continually regenerates ozone in the atmosphere and converts ultraviolet radiation (UV) into heat
- Water cycle - moves water continuously on, above and below the surface shifting between states of liquid, solution, ice and vapour
- Methane cycle - moves methane between geological and biogeochemical sources and reactions in the atmosphere
- Hydrogen cycle - a biogeochemical cycle brought about by a combination of biological and abiological processes
- Phosphorus cycle - the movement of phosphorus through the lithosphere, hydrosphere, and biosphere
- Sulfur cycle - a biogeochemical process resulting form the mineralization of organic sulfur, oxidation, reduction and incorporation into organic compounds
- Carbonate–silicate cycle transforms silicate rocks to carbonate rocks by weathering and sedimentation and transforms carbonate rocks back into silicates by metamorphism and magmatism.[8]
- Rock cycle - switches rock between its three forms: sedimentary, metamorphic, and igneous
- Mercury cycle - a biogeochemical process in which naturally occurring mercury is bioaccumulated before recombining with sulfur and returning to geological sources as sediments
Other chemical cycles include hydrogen peroxide.[9]
Mars

Recent evidence suggests that similar chemical cycles to Earth's occur on a lesser scale on Mars, facilitated by the thin atmosphere, including possible carbon,[10] water,[11] sulphur,[12] methane,[13] oxygen,[14] ozone,[15] and nitrogen[16] cycles. Many studies point to significantly more active chemical cycles on Mars in the past, however the faint young Sun paradox has proved problematic in determining chemical cycles involved in early climate models of the planet.[17]
Jupiter

Recent studies indicate water-ammonia (hyrdological) and hydrogen sulfide cycles on Jupiter.[18]
Significant chemical cycles exist on Jupiter's moons. Recent evidence points to Europa possessing several active cycles, most notably a water cycle.[19] Other studies suggest an oxygen and carbon dioxide cycle.[20] Io and Europa, appear to have sulphur cycles involving their lithospheres.[21] In addition, the Io plasma torus contributes to a sulphur cycle on Jupiter and Ganymede.[22] Studies also imply active oxygen cycles on Ganymede and Callisto.[23]
Saturn

Some studies suggest an ammonia cycle similar to Jupiter's also operates on Saturn in addition to chemical cycles induced by photolysis.[24]
The cycles of its moons are of particular interest. Observations by Cassini–Huygens of Titan's atmosphere and interactions with its liquid mantle give rise to has several active chemical cycles including a methane,[25] hydrocarbon,[26] hydrogen,[27] and carbon[28] cycles. Enceladus has an active hydrological, silicate and possibly a nitrogen cycle.[29][30]
Uranus
Uranus has an active methane cycle.[31] Methane is converted to hydrocarbons through photolysis which condenses and as they are heated, release methane which rises to the upper atmosphere.
Studies by Grundy et al. (2006) indicate active carbon cycles operates on Titania, Umbriel and Ariel and Oberon through the ongoing sublimation and deposition of carbon dioxide, though some is lost to space over long periods of time.[32]
Neptune
Neptune's internal heat and convection drives a carbon cycle.[33]
Models predicted the presence of seasonal nitrogen cycles on the moon Triton,[34] however this has not been supported by observations to date.
Pluto-Charon system
Models predict a seaonal nitrogen cycle on Pluto[35] and observations by New Horizons appear to support this.
References
- ^ Vladimir E. Fortov (26 December 2015). Extreme States of Matter: High Energy Density Physics. Springer. pp. 97–. ISBN 978-3-319-18953-6.
- ^ Palouš, Jan (2007). "Star – Gas Cycle in Galaxies". Proceedings of the International Astronomical Union. 2 (S235): 268. doi:10.1017/S1743921306006569. ISSN 1743-9213.
- ^ Mills, Franklin P.; Allen, Mark (2007). "A review of selected issues concerning the chemistry in Venus' middle atmosphere". Planetary and Space Science. 55 (12): 1729–1740. doi:10.1016/j.pss.2007.01.012. ISSN 0032-0633.
- ^ Nick Strobel. "Venus". Retrieved 17 February 2009.
- ^ Jessup, Kandis Lea; Marcq, Emmanuel; Mills, Franklin; Mahieux, Arnaud; Limaye, Sanjay; Wilson, Colin; Allen, Mark; Bertaux, Jean-Loup; Markiewicz, Wojciech; Roman, Tony; Vandaele, Ann-Carine; Wilquet, Valerie; Yung, Yuk (2015). "Coordinated Hubble Space Telescope and Venus Express Observations of Venus' upper cloud deck". Icarus. 258: 309–336. doi:10.1016/j.icarus.2015.05.027. ISSN 0019-1035.
- ^ Zhang, Xi; Liang, Mao-Chang; Montmessin, Franck; Bertaux, Jean-Loup; Parkinson, Christopher; Yung, Yuk L. (2010). "Photolysis of sulphuric acid as the source of sulphur oxides in the mesosphere of Venus". Nature Geoscience. 3 (12): 834–837. doi:10.1038/ngeo989. ISSN 1752-0894.
- ^ Montmessin, F.; Bertaux, J.-L.; Lefèvre, F.; Marcq, E.; Belyaev, D.; Gérard, J.-C.; Korablev, O.; Fedorova, A.; Sarago, V.; Vandaele, A.C. (2011). "A layer of ozone detected in the nightside upper atmosphere of Venus". Icarus. 216 (1): 82–85. doi:10.1016/j.icarus.2011.08.010. ISSN 0019-1035.
- ^ Berner, Robert; Lasaga, Antonio; Garrels, Robert (September 1983). "The Carbonate-Silicate Geochemical Cycle and its Effect on Atmospheric Carbon Dioxide over the Past 100 Million Years" (PDF). American Journal of Science. 283: 641–683. Retrieved Feb 3, 2015.
- ^ Allen, Nicholas D.C.; González Abad, Gonzalo; Bernath, Peter F.; Boone, Chris D. (2013). "Satellite observations of the global distribution of hydrogen peroxide (H2O2) from ACE". Journal of Quantitative Spectroscopy and Radiative Transfer. 115: 66–77. doi:10.1016/j.jqsrt.2012.09.008. ISSN 0022-4073.
- ^ Edwards, Christopher S.; Ehlmann, Bethany L. (2015). "Carbon sequestration on Mars". Geology. 43 (10): 863–866. doi:10.1130/G36983.1. ISSN 0091-7613.
- ^ Machtoub, G. (2012). "Modeling the hydrological cycle on Mars". Journal of Advances in Modeling Earth Systems. 4. doi:10.1029/2011MS000069. ISSN 1942-2466.
- ^ King, P. L.; McLennan, S. M. (2010). "Sulfur on Mars". Elements. 6 (2): 107–112. doi:10.2113/gselements.6.2.107. ISSN 1811-5209.
- ^ Wray, James J.; Ehlmann, Bethany L. (2011). "Geology of possible Martian methane source regions". Planetary and Space Science. 59 (2–3): 196–202. doi:10.1016/j.pss.2010.05.006. ISSN 0032-0633.
- ^ Farquhar, James; Thiemens, Mark H. (2000). "Oxygen cycle of the Martian atmosphere-regolith system: Δ17O of secondary phases in Nakhla and Lafayette". Journal of Geophysical Research: Planets. 105 (E5): 11991–11997. doi:10.1029/1999JE001194. ISSN 0148-0227.
- ^ Montmessin, Franck; Lefèvre, Franck (2013). "Transport-driven formation of a polar ozone layer on Mars". Nature Geoscience. 6 (11): 930–933. doi:10.1038/ngeo1957. ISSN 1752-0894.
- ^ Boxe, C.S.; Hand, K.P.; Nealson, K.H.; Yung, Y.L.; Saiz-Lopez, A. (2012). "An active nitrogen cycle on Mars sufficient to support a subsurface biosphere". International Journal of Astrobiology. 11 (02): 109–115. doi:10.1017/S1473550411000401. ISSN 1473-5504.
- ^ Wordsworth, R.; Forget, F.; Millour, E.; Head, J.W.; Madeleine, J.-B.; Charnay, B. (2013). "Global modelling of the early martian climate under a denser CO2 atmosphere: Water cycle and ice evolution". Icarus. 222 (1): 1–19. doi:10.1016/j.icarus.2012.09.036. ISSN 0019-1035.
- ^ Palotai, Csaba; Dowling, Timothy E.; Fletcher, Leigh N. (2014). "3D Modeling of interactions between Jupiter's ammonia clouds and large anticyclones". Icarus. 232: 141–156. doi:10.1016/j.icarus.2014.01.005. ISSN 0019-1035.
- ^ Kattenhorn, Simon A.; Prockter, Louise M. (2014). "Evidence for subduction in the ice shell of Europa". Nature Geoscience. 7 (10): 762–767. doi:10.1038/ngeo2245. ISSN 1752-0894.
- ^ Hand, Kevin P.; Chyba, Christopher F.; Carlson, Robert W.; Cooper, John F. (2006). "Clathrate Hydrates of Oxidants in the Ice Shell of Europa". Astrobiology. 6 (3): 463–482. doi:10.1089/ast.2006.6.463. ISSN 1531-1074.
- ^ Battaglia, Steven M.; Stewart, Michael A.; Kieffer, Susan W. (June 2014). "Io's theothermal (sulfur) - Lithosphere cycle inferred from sulfur solubility modeling of Pele's magma supply". Icarus. 235: 123–129. Bibcode:2014Icar.235.123B. doi:10.1016/j.icarus.2014.03.019.
{{cite journal}}: Check|bibcode=length (help) - ^ Cheng, Andrew F. (1984). "Escape of sulfur and oxygen from Io". Journal of Geophysical Research. 89 (A6): 3939. doi:10.1029/JA089iA06p03939. ISSN 0148-0227.
- ^ Vidal RA, Bahr D, Baragiola RA, Peters M. Oxygen on Ganymede: laboratory studies. Science. 1997;276(5320):1839-42.
- ^ West, R. A.; Baines, K. H.; Karkoschka, E.; Sánchez-Lavega, A. (2009). "Clouds and Aerosols in Saturn's Atmosphere": 161–179. doi:10.1007/978-1-4020-9217-6_7.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Atreya, Sushil K.; Adams, Elena Y.; Niemann, Hasso B.; Demick-Montelara, Jaime E.; Owen, Tobias C.; Fulchignoni, Marcello; Ferri, Francesca; Wilson, Eric H. (2006). "Titan's methane cycle". Planetary and Space Science. 54 (12): 1177–1187. doi:10.1016/j.pss.2006.05.028. ISSN 0032-0633.
- ^ Tobie, G; Choukroun, M; Grasset, O; Le Mouelic, S; Lunine, J.I; Sotin, C; Bourgeois, O; Gautier, D; Hirtzig, M; Lebonnois, S; Le Corre, L (2009). "Evolution of Titan and implications for its hydrocarbon cycle". Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 367 (1889): 617–631. doi:10.1098/rsta.2008.0246. ISSN 1364-503X.
- ^ Lebonnois, S.ébastien; Bakes, E.L.O.; McKay, Christopher P. (2003). "Atomic and molecular hydrogen budget in Titan's atmosphere". Icarus. 161 (2): 474–485. doi:10.1016/S0019-1035(02)00039-8. ISSN 0019-1035.
- ^ Choukroun, M.; Sotin, C. (2012). "Is Titan's shape caused by its meteorology and carbon cycle?". Geophysical Research Letters. 39 (4): n/a–n/a. doi:10.1029/2011GL050747. ISSN 0094-8276.
- ^ Parkinson, C. D.; Liang, M.-C.; Hartman, H.; Hansen, C. J.; Tinetti, G.; Meadows, V.; Kirschvink, J. L.; Yung, Y. L. (2007). "Enceladus: Cassini observations and implications for the search for life". Astronomy and Astrophysics. 463 (1): 353–357. doi:10.1051/0004-6361:20065773. ISSN 0004-6361.
- ^ Parkinson, Christopher D.; Liang, Mao-Chang; Yung, Yuk L.; Kirschivnk, Joseph L. (2008). "Habitability of Enceladus: Planetary Conditions for Life". Origins of Life and Evolution of Biospheres. 38 (4): 355–369. doi:10.1007/s11084-008-9135-4. ISSN 0169-6149.
- ^ Richard Schmude, Jr. (29 June 2009). Uranus, Neptune, and Pluto and How to Observe Them. Springer Science & Business Media. pp. 67–. ISBN 978-0-387-76602-7.
- ^ Grundy, W. M.; Young, L. A.; Spencer, J. R.; Johnson, R. E.; Young, E. F.; Buie, M. W. (October 2006). "Distributions of H2O and CO2 ices on Ariel, Umbriel, Titania, and Oberon from IRTF/SpeX observations". Icarus. 184 (2): 543–555. arXiv:0704.1525. Bibcode:2006Icar..184..543G. doi:10.1016/j.icarus.2006.04.016.
- ^ Dale P. Cruikshank; Mildred Shapley Matthews; A. M. Schumann (1995). Neptune and Triton. University of Arizona Press. pp. 500–. ISBN 978-0-8165-1525-7.
- ^ Hansen, Candice J.; Paige, David A. (1992). "A thermal model for the seasonal nitrogen cycle on Triton". Icarus. 99 (2): 273–288. doi:10.1016/0019-1035(92)90146-X. ISSN 0019-1035.
- ^ Hansen, Candice J.; Paige, David A. (1996). "Seasonal Nitrogen Cycles on Pluto". Icarus. 120 (2): 247–265. doi:10.1006/icar.1996.0049. ISSN 0019-1035.
