X-inactivation: Difference between revisions
clarification |
consistent citation formatting and templated citations |
||
| Line 2: | Line 2: | ||
[[Image:6-year old tortoise shell cat.jpg|thumb|right|The coloration of [[Tortoiseshell cat|tortoiseshell]] and [[calico cat]]s is a visible manifestation of X-inactivation. The black and orange [[alleles]] of a fur coloration gene reside on the X chromosome. For any given patch of fur, the inactivation of an X chromosome that carries one gene results in the fur color of the other, active gene.]] |
[[Image:6-year old tortoise shell cat.jpg|thumb|right|The coloration of [[Tortoiseshell cat|tortoiseshell]] and [[calico cat]]s is a visible manifestation of X-inactivation. The black and orange [[alleles]] of a fur coloration gene reside on the X chromosome. For any given patch of fur, the inactivation of an X chromosome that carries one gene results in the fur color of the other, active gene.]] |
||
[[Image:Sd4hi-unten-crop.jpg|thumb|Nucleus of a female cell. Top: Both X-chromosomes are detected, by [[Fluorescence in situ hybridization|FISH]]. Bottom: The same nucleus stained with a DNA stain ([[DAPI]]). The Barr body is indicated by the arrow, it identifies the inactive X (Xi).]] |
[[Image:Sd4hi-unten-crop.jpg|thumb|Nucleus of a female cell. Top: Both X-chromosomes are detected, by [[Fluorescence in situ hybridization|FISH]]. Bottom: The same nucleus stained with a DNA stain ([[DAPI]]). The Barr body is indicated by the arrow, it identifies the inactive X (Xi).]] |
||
[[Image:BarrBodyBMC Biology2-21-Fig1clip293px.jpg|thumb|An interphase female human fibroblast cell.<ref>{{cite journal |vauthors=Gartler SM, Varadarajan KR, Luo P, Canfield TK, Traynor J, Francke U, Hansen RS | |
[[Image:BarrBodyBMC Biology2-21-Fig1clip293px.jpg|thumb|An interphase female human fibroblast cell.<ref>{{cite journal | vauthors = Gartler SM, Varadarajan KR, Luo P, Canfield TK, Traynor J, Francke U, Hansen RS | title = Normal histone modifications on the inactive X chromosome in ICF and Rett syndrome cells: implications for methyl-CpG binding proteins | journal = BMC Biology | volume = 2 | issue = | pages = 21 | date = September 2004 | pmid = 15377381 | pmc = 521681 | doi = 10.1186/1741-7007-2-21 | url = http://www.biomedcentral.com/1741-7007/2/21 }}</ref> Arrows point to sex chromatin on DNA ([[DAPI]]) in cell nucleus(left), and to the corresponding X chromatin (right).<br />Left: DNA (DAPI)-stained nucleus. Arrow indicates the location of Barr body(Xi). Right: DNA associated [[histone]]s protein detected]] |
||
[[Image:XistRNADNAFISH.jpg|thumb|right|The figure shows [[confocal microscopy]] images from a combined RNA-DNA [[Fluorescence in situ hybridization|FISH]] experiment for [[Xist]] in fibroblast cells from adult female mouse, demonstrating that Xist RNA is coating only one of the X-chromosomes. RNA FISH signals from Xist RNA are shown in red color, marking the inactive X-chromosome (Xi). DNA FISH signals from Xist loci are shown in yellow color, marking both active and inactive X-chromosomes (Xa, Xi). The nucleus ([[DAPI]]-stained) is shown in blue color. The figure is adapted from:.<ref name="pmid21047393"/>]] |
[[Image:XistRNADNAFISH.jpg|thumb|right|The figure shows [[confocal microscopy]] images from a combined RNA-DNA [[Fluorescence in situ hybridization|FISH]] experiment for [[Xist]] in fibroblast cells from adult female mouse, demonstrating that Xist RNA is coating only one of the X-chromosomes. RNA FISH signals from Xist RNA are shown in red color, marking the inactive X-chromosome (Xi). DNA FISH signals from Xist loci are shown in yellow color, marking both active and inactive X-chromosomes (Xa, Xi). The nucleus ([[DAPI]]-stained) is shown in blue color. The figure is adapted from:.<ref name="pmid21047393"/>]] |
||
| Line 8: | Line 8: | ||
==History== |
==History== |
||
In 1959 [[Susumu Ohno]] showed that the two X-chromosomes of mammals were different: one appeared similar to the [[autosomes]]; the other was condensed and heterochromatic.<ref>{{cite journal |vauthors=Ohno S, Kaplan WD, Kinosita R | |
In 1959 [[Susumu Ohno]] showed that the two X-chromosomes of mammals were different: one appeared similar to the [[autosomes]]; the other was condensed and heterochromatic.<ref>{{cite journal | vauthors = Ohno S, Kaplan WD, Kinosita R | title = Formation of the sex chromatin by a single X-chromosome in liver cells of Rattus norvegicus | journal = Experimental Cell Research | volume = 18 | issue = 2 | pages = 415–8 | date = October 1959 | pmid = 14428474 | doi = 10.1016/0014-4827(59)90031-X }}</ref> This finding suggested, independently to two groups of investigators, that one of the X-chromosomes underwent inactivation. In 1961, [[Mary F. Lyon|Mary Lyon]] proposed the random inactivation of one female X chromosome to explain the mottled phenotype of female mice [[heterozygous]] for coat color [[gene]]s.<ref name="Lyon 1961">{{cite journal | vauthors = Lyon MF | title = Gene action in the X-chromosome of the mouse (Mus musculus L.) | journal = Nature | volume = 190 | issue = 4773 | pages = 372–3 | date = April 1961 | pmid = 13764598 | doi = 10.1038/190372a0 }}</ref> The Lyon hypothesis also accounted for the findings that one copy of the X chromosome in female cells was highly condensed, and that mice with only one copy of the X chromosome developed as infertile females. This suggested<ref>{{cite journal | vauthors = Beutler E | title = Glucose-6-phosphate dehydrogenase deficiency: a historical perspective | journal = Blood | volume = 111 | issue = 1 | pages = 16–24 | date = January 2008 | pmid = 18156501 | doi = 10.1182/blood-2007-04-077412 }}</ref> to [[Ernest Beutler]], studying heterozygous females for [[Glucose-6-phosphate dehydrogenase]] (G6PD) deficiency, that there were two red cell populations of erythrocytes in such heterozygotes: deficient cells and normal cells,<ref>{{cite journal | vauthors = Beutler E, Yeh M, Fairbanks VF | title = The normal human female as a mosaic of X-chromosome activity: studies using the gene for C-6-PD-deficiency as a marker | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 48 | issue = 1 | pages = 9–16 | date = January 1962 | pmid = 13868717 | pmc = 285481 | doi = 10.1073/pnas.48.1.9 }}</ref> depending on whether the inactivated X chromosome (in the nucleus of the red cell's precursor cell) contains the normal or defective G6PD allele. |
||
==Mechanism== |
==Mechanism== |
||
=== Cycle of X chromosome activation === |
=== Cycle of X chromosome activation === |
||
X-inactivation is part of the activation cycle of the X chromosome throughout the female life. The egg, and the fertilized zygote initially uses maternal transcripts, and the whole embryonic genome is silenced, which lasts until lasting until zygotic genome activation. Thereafter, all mouse cells undergo an early, [[Imprinting (genetics)|imprinted]] inactivation of the paternally-derived X chromosome in [[mammalian embryogenesis|4-8 cell stage]] [[embryo]]s <ref>{{cite journal |vauthors=Takagi N, Sasaki M | |
X-inactivation is part of the activation cycle of the X chromosome throughout the female life. The egg, and the fertilized zygote initially uses maternal transcripts, and the whole embryonic genome is silenced, which lasts until lasting until zygotic genome activation. Thereafter, all mouse cells undergo an early, [[Imprinting (genetics)|imprinted]] inactivation of the paternally-derived X chromosome in [[mammalian embryogenesis|4-8 cell stage]] [[embryo]]s <ref>{{cite journal | vauthors = Takagi N, Sasaki M | title = Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse | journal = Nature | volume = 256 | issue = 5519 | pages = 640–2 | date = August 1975 | pmid = 1152998 | doi = 10.1038/256640a0 }}</ref><ref>{{cite journal | vauthors = Cheng MK, Disteche CM | title = Silence of the fathers: early X inactivation | journal = BioEssays | volume = 26 | issue = 8 | pages = 821–4 | date = August 2004 | pmid = 15273983 | doi = 10.1002/bies.20082 | url = http://www3.interscience.wiley.com/cgi-bin/fulltext/109565168/PDFSTART }}</ref><ref name="okamoto">{{cite journal | vauthors = Okamoto I, Otte AP, Allis CD, Reinberg D, Heard E | title = Epigenetic dynamics of imprinted X inactivation during early mouse development | journal = Science | volume = 303 | issue = 5658 | pages = 644–9 | date = January 2004 | pmid = 14671313 | doi = 10.1126/science.1092727 }}</ref><ref name=":2">{{cite journal | vauthors = Deng Q, Ramsköld D, Reinius B, Sandberg R | title = Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells | journal = Science | volume = 343 | issue = 6167 | pages = 193–6 | date = January 2014 | pmid = 24408435 | doi = 10.1126/science.1245316 }}</ref><ref name=":3">{{cite journal | vauthors = Borensztein M, Syx L, Ancelin K, Diabangouaya P, Picard C, Liu T, Liang JB, Vassilev I, Galupa R, Servant N, Barillot E, Surani A, Chen CJ, Heard E | title = Xist-dependent imprinted X inactivation and the early developmental consequences of its failure | language = En | journal = Nature Structural & Molecular Biology | volume = 24 | issue = 3 | pages = 226–233 | date = March 2017 | pmid = 28134930 | pmc = 5337400 | doi = 10.1038/nsmb.3365 | url = http://www.nature.com/articles/nsmb.3365 }}</ref>. The [[extraembryonic tissue]]s (which give rise to the [[placenta]] and other tissues supporting the embryo) retain this early imprinted inactivation, and thus only the maternal X chromosome is active in these tissues. |
||
In the early [[blastocyst]], this initial, imprinted X-inactivation is reversed in the cells of the [[inner cell mass]] (which give rise to the embryo), and in these cells both X chromosomes become active again. Each of these cells then independently and randomly inactivates one copy of the X chromosome.<ref name=okamoto/> This inactivation event is irreversible during the lifetime of the individual, with the exception of the germline. In the female [[germline]] before meiotic entry, X-inactivation is reversed, so that after meiosis all haploid [[oocyte]]s contain an active X chromosome. |
In the early [[blastocyst]], this initial, imprinted X-inactivation is reversed in the cells of the [[inner cell mass]] (which give rise to the embryo), and in these cells both X chromosomes become active again. Each of these cells then independently and randomly inactivates one copy of the X chromosome.<ref name=okamoto/> This inactivation event is irreversible during the lifetime of the individual, with the exception of the germline. In the female [[germline]] before meiotic entry, X-inactivation is reversed, so that after meiosis all haploid [[oocyte]]s contain an active X chromosome. |
||
| Line 41: | Line 41: | ||
|1 |
|1 |
||
|Zygotic genome activation |
|Zygotic genome activation |
||
|2-4 cell stage<ref name=":4">{{ |
|2-4 cell stage<ref name=":4">{{cite journal | vauthors = Xue Z, Huang K, Cai C, Cai L, Jiang CY, Feng Y, Liu Z, Zeng Q, Cheng L, Sun YE, Liu JY, Horvath S, Fan G | title = Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing | language = En | journal = Nature | volume = 500 | issue = 7464 | pages = 593–7 | date = August 2013 | pmid = 23892778 | pmc = 4950944 | doi = 10.1038/nature12364 }}</ref> |
||
|2-8 cell stage<ref name=":4" /> |
|2-8 cell stage<ref name=":4" /> |
||
|- |
|- |
||
| Line 47: | Line 47: | ||
|Imprinted (paternal) X-inactivation |
|Imprinted (paternal) X-inactivation |
||
|4-8 cell stage<ref name=":2" /><ref name=":3" /> |
|4-8 cell stage<ref name=":2" /><ref name=":3" /> |
||
|Unclear if it takes place in humans<ref name=":5">{{ |
|Unclear if it takes place in humans<ref name=":5">{{cite journal | vauthors = Deng X, Berletch JB, Nguyen DK, Disteche CM | title = X chromosome regulation: diverse patterns in development, tissues and disease | language = En | journal = Nature Reviews. Genetics | volume = 15 | issue = 6 | pages = 367–78 | date = June 2014 | pmid = 24733023 | pmc = 4117651 | doi = 10.1038/nrg3687 }}</ref> |
||
|- |
|- |
||
|3 |
|3 |
||
| Line 62: | Line 62: | ||
|X-reactivation in primordial germ cells before meiosis |
|X-reactivation in primordial germ cells before meiosis |
||
| |
| |
||
|From before developmental week 4 up to week 14<ref>{{ |
|From before developmental week 4 up to week 14<ref>{{cite journal | vauthors = Vértesy Á, Arindrarto W, Roost MS, Reinius B, Torrens-Juaneda V, Bialecka M, Moustakas I, Ariyurek Y, Kuijk E, Mei H, Sandberg R, van Oudenaarden A, Chuva de Sousa Lopes SM | title = Parental haplotype-specific single-cell transcriptomics reveal incomplete epigenetic reprogramming in human female germ cells | language = En | journal = Nature Communications | volume = 9 | issue = 1 | pages = 1873 | date = May 2018 | pmid = 29760424 | pmc = 5951918 | doi = 10.1038/s41467-018-04215-7 }}</ref><ref>{{cite journal | vauthors = Guo F, Yan L, Guo H, Li L, Hu B, Zhao Y, Yong J, Hu Y, Wang X, Wei Y, Wang W, Li R, Yan J, Zhi X, Zhang Y, Jin H, Zhang W, Hou Y, Zhu P, Li J, Zhang L, Liu S, Ren Y, Zhu X, Wen L, Gao YQ, Tang F, Qiao J | title = The Transcriptome and DNA Methylome Landscapes of Human Primordial Germ Cells | journal = Cell | volume = 161 | issue = 6 | pages = 1437–52 | date = June 2015 | pmid = 26046443 | doi = 10.1016/j.cell.2015.05.015 }}</ref> |
||
|} |
|} |
||
====== Inheritance of inactivation status across cell generations ====== |
====== Inheritance of inactivation status across cell generations ====== |
||
The descendants of each cell which inactivated a particular X chromosome will also inactivate that same chromosome. This phenomenon, which can be observed in the coloration of [[tortoiseshell cat]]s when females are [[heterozygous]] for the [[sex linkage|X-linked gene]], should not be confused with [[mosaic (genetics)|mosaicism]], which is a term that specifically refers to differences in the [[genotype]] of various cell populations in the same individual; X-inactivation, which is an [[epigenetics|epigenetic]] change that results in a different phenotype, is ''not'' a change at the [[genotype|genotypic]] level. For an individual cell or lineage the inactivation is therefore [[Skewed X-inactivation|skewed]] or '[[Skewed X-inactivation|non-random]]', and this can give rise to mild symptoms in female 'carriers' of [[X-linked]] genetic disorders.<ref>{{cite journal |title=X |
The descendants of each cell which inactivated a particular X chromosome will also inactivate that same chromosome. This phenomenon, which can be observed in the coloration of [[tortoiseshell cat]]s when females are [[heterozygous]] for the [[sex linkage|X-linked gene]], should not be confused with [[mosaic (genetics)|mosaicism]], which is a term that specifically refers to differences in the [[genotype]] of various cell populations in the same individual; X-inactivation, which is an [[epigenetics|epigenetic]] change that results in a different phenotype, is ''not'' a change at the [[genotype|genotypic]] level. For an individual cell or lineage the inactivation is therefore [[Skewed X-inactivation|skewed]] or '[[Skewed X-inactivation|non-random]]', and this can give rise to mild symptoms in female 'carriers' of [[X-linked]] genetic disorders.<ref>{{cite journal | vauthors = Puck JM, Willard HF | title = X inactivation in females with X-linked disease | journal = The New England Journal of Medicine | volume = 338 | issue = 5 | pages = 325–8 | date = January 1998 | pmid = 9445416 | doi = 10.1056/NEJM199801293380611 }}</ref> |
||
===Selection of one active X chromosome=== |
===Selection of one active X chromosome=== |
||
Normal females possess two X chromosomes, and in any given cell one chromosome will be active (designated as Xa) and one will be inactive (Xi). However, studies of individuals with [[X chromosome#Role in diseases|extra copies of the X chromosome]] show that in cells with more than two X chromosomes there is still only one Xa, and all the remaining X chromosomes are inactivated. This indicates that the default state of the X chromosome in females is inactivation, but one X chromosome is always selected to remain active. |
Normal females possess two X chromosomes, and in any given cell one chromosome will be active (designated as Xa) and one will be inactive (Xi). However, studies of individuals with [[X chromosome#Role in diseases|extra copies of the X chromosome]] show that in cells with more than two X chromosomes there is still only one Xa, and all the remaining X chromosomes are inactivated. This indicates that the default state of the X chromosome in females is inactivation, but one X chromosome is always selected to remain active. |
||
It is understood that X-chromosome inactivation is a random process, occurring at about the time of [[gastrulation]] in the [[epiblast]] (cells that will give rise to the embryo). The maternal and paternal X chromosomes have an equal probability of inactivation. This would suggest that women would be expected to suffer from X-linked disorders approximately 50% as often as men (because women have two X chromosomes, while men have only one); however, in actuality, the occurrence of these disorders in females is much lower than that. One explanation for this disparity is that 12–20% <ref>{{cite journal| |
It is understood that X-chromosome inactivation is a random process, occurring at about the time of [[gastrulation]] in the [[epiblast]] (cells that will give rise to the embryo). The maternal and paternal X chromosomes have an equal probability of inactivation. This would suggest that women would be expected to suffer from X-linked disorders approximately 50% as often as men (because women have two X chromosomes, while men have only one); however, in actuality, the occurrence of these disorders in females is much lower than that. One explanation for this disparity is that 12–20% <ref>{{cite journal | vauthors = Balaton BP, Cotton AM, Brown CJ | title = Derivation of consensus inactivation status for X-linked genes from genome-wide studies | journal = Biology of Sex Differences | volume = 6 | issue = 35 | pages = 35 | date = 30 December 2015 | pmid = 26719789 | doi = 10.1186/s13293-015-0053-7 | url = https://doi.org/10.1186/s13293-015-0053-7 }}</ref> of genes on the inactivated X chromosome remain expressed, thus providing women with added protection against defective genes coded by the X-chromosome. Some{{who|date=October 2014}} suggest that this disparity must be evidence of preferential (non-random) inactivation. Preferential inactivation of the paternal X-chromosome occurs in both marsupials and in cell lineages that form the membranes surrounding the embryo,<ref>{{cite journal | vauthors = Graves JA | title = Mammals that break the rules: genetics of marsupials and monotremes | journal = Annual Review of Genetics | volume = 30 | pages = 233–60 | year = 1996 | pmid = 8982455 | doi = 10.1146/annurev.genet.30.1.233 }}</ref> whereas in placental mammals either the maternally or the paternally derived X-chromosome may be inactivated in different cell lines.<ref>{{cite journal | vauthors = Lyon MF | title = X-chromosome inactivation and developmental patterns in mammals | journal = Biological Reviews of the Cambridge Philosophical Society | volume = 47 | issue = 1 | pages = 1–35 | date = January 1972 | pmid = 4554151 | doi = 10.1111/j.1469-185X.1972.tb00969.x }}</ref> |
||
The time period for X-chromosome inactivation explains this disparity. Inactivation occurs in the epiblast during gastrulation, which gives rise to the embryo.<ref>{{cite web| |
The time period for X-chromosome inactivation explains this disparity. Inactivation occurs in the epiblast during gastrulation, which gives rise to the embryo.<ref>{{cite web| vauthors = Migeon, B |title=X chromosome inactivation in human cells|url=http://hstalks.com/main/view_talk.php?t=1676&r=494&c=252|work=The Biomedical & Life Sciences Collection|publisher=Henry Stewart Talks, Ltd|access-date=15 December 2013 |pages=1–54|year=2010}}</ref> Inactivation occurs on a cellular level, resulting in a mosaic expression, in which patches of cells have an inactive maternal X-chromosome, while other patches have an inactive paternal X-chromosome. For example, a female heterozygous for haemophilia (an X-linked disease) would have about half of her liver cells functioning properly, which is typically enough to ensure normal blood clotting.<ref name="Gartler_2001">{{cite journal | last1 = Gartler | first1 = Stanley M | last2 = Goldman | first2 = Michael A | name-list-format = vanc | title = X-Chromosome Inactivation | url = http://web.udl.es/usuaris/e4650869/docencia/segoncicle/genclin98/recursos_classe_(pdf)/revisionsPDF/XChromoInac.pdf | work = Encyclopedia of Life Sciences | publisher = Nature Publishing Group | pages = 1–2 | year=2001 }}</ref><ref name="Connallon_2013">{{cite journal | vauthors = Connallon T, Clark AG | title = Sex-differential selection and the evolution of X inactivation strategies | journal = PLoS Genetics | volume = 9 | issue = 4 | pages = e1003440 | date = April 2013 | pmid = 23637618 | pmc = 3630082 | doi = 10.1371/journal.pgen.1003440 }}</ref> Chance could result in significantly more dysfunctional cells; however, such statistical extremes are unlikely. Genetic differences on the chromosome may also render one X-chromosome more likely to undergo inactivation. Also, if one X-chromosome has a mutation hindering its growth or rendering it non viable, cells which randomly inactivated that X will have a selective advantage over cells which randomly inactivated the normal allele. Thus, although inactivation is initially random, cells that inactivate a normal allele (leaving the mutated allele active) will eventually be overgrown and replaced by functionally normal cells in which nearly all have the same X-chromosome activated.<ref name="Gartler_2001"/> |
||
It is hypothesized{{by whom|date=October 2014}} that there is an autosomally-encoded 'blocking factor' which binds to the X chromosome and prevents its inactivation. The model postulates that there is a limiting blocking factor, so once the available blocking factor molecule binds to one X chromosome the remaining X chromosome(s) are not protected from inactivation. This model is supported by the existence of a single Xa in cells with many X chromosomes and by the existence of two active X chromosomes in cell lines with twice the normal number of autosomes.<ref>{{cite |
It is hypothesized{{by whom|date=October 2014}} that there is an autosomally-encoded 'blocking factor' which binds to the X chromosome and prevents its inactivation. The model postulates that there is a limiting blocking factor, so once the available blocking factor molecule binds to one X chromosome the remaining X chromosome(s) are not protected from inactivation. This model is supported by the existence of a single Xa in cells with many X chromosomes and by the existence of two active X chromosomes in cell lines with twice the normal number of autosomes.<ref>{{cite book | first1 = Tahsin Stefan | last1 = Barakat | first2 = Joost | last2 = Gribnau | chapter = X Chromosome Inactivation and Embryonic Stem Cells | chapter-url = https://www.ncbi.nlm.nih.gov/books/NBK45037/ | title = The Cell Biology of Stem Cells | editor-first1 = Eran | editor-last1 = Meshorer | editor-first2 = Kathrin | editor-last2 = Plath | year = 2010 | publisher = Landes Bioscience and Springer Science+Business Media }}</ref> |
||
Sequences at the '''X inactivation center''' ('''XIC'''), present on the X chromosome, control the silencing of the X chromosome. The hypothetical blocking factor is predicted to bind to sequences within the XIC. |
Sequences at the '''X inactivation center''' ('''XIC'''), present on the X chromosome, control the silencing of the X chromosome. The hypothetical blocking factor is predicted to bind to sequences within the XIC. |
||
| Line 82: | Line 82: | ||
It’s easy to see the effect of female X heterozygosity in localized traits, such as the unique coat pattern of a calico cat. It can be more difficult, however, to fully understand the expression of un-localized traits in these females, such as the expression of disease. |
It’s easy to see the effect of female X heterozygosity in localized traits, such as the unique coat pattern of a calico cat. It can be more difficult, however, to fully understand the expression of un-localized traits in these females, such as the expression of disease. |
||
Since males only have one copy of the X chromosome, all expressed X-chromosomal [[gene]]s (or [[allele]]s, in the case of multiple variant forms for a given gene in the population) are located on that copy of the chromosome. Females, however, will primarily express the genes or alleles located on the X-chromosomal copy that remains active. Considering the situation for one gene or multiple genes causing individual differences in a particular [[Phenotypic trait|phenotype]] (i.e., causing variation observed in the population for that phenotype), in homozygous females it doesn’t particularly matter which copy of the chromosome is inactivated, as the alleles on both copies are the same. However, in females that are heterozygous at the causal genes, the inactivation of one copy of the chromosome over the other can have a direct impact on their phenotypic value. Because of this phenomenon, there is an observed increase in phenotypic variation in females that are heterozygous at the involved gene or genes than in females that are homozygous at that gene or those genes.<ref>{{ |
Since males only have one copy of the X chromosome, all expressed X-chromosomal [[gene]]s (or [[allele]]s, in the case of multiple variant forms for a given gene in the population) are located on that copy of the chromosome. Females, however, will primarily express the genes or alleles located on the X-chromosomal copy that remains active. Considering the situation for one gene or multiple genes causing individual differences in a particular [[Phenotypic trait|phenotype]] (i.e., causing variation observed in the population for that phenotype), in homozygous females it doesn’t particularly matter which copy of the chromosome is inactivated, as the alleles on both copies are the same. However, in females that are heterozygous at the causal genes, the inactivation of one copy of the chromosome over the other can have a direct impact on their phenotypic value. Because of this phenomenon, there is an observed increase in phenotypic variation in females that are heterozygous at the involved gene or genes than in females that are homozygous at that gene or those genes.<ref>{{cite journal | vauthors = Ma L, Hoffman G, Keinan A | title = X-inactivation informs variance-based testing for X-linked association of a quantitative trait | journal = BMC Genomics | volume = 16 | pages = 241 | date = March 2015 | pmid = 25880738 | pmc = 4381508 | doi = 10.1186/s12864-015-1463-y | url = http://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-015-1463-y }}</ref> There are many different ways in which the phenotypic variation can play out. In many cases, heterozygous females may be asymptomatic or only present minor symptoms of a given disorder, such as with [[Adrenoleukodystrophy|X-linked adrenoleukodystrophy.]]<ref>{{cite journal | vauthors = Habekost CT, Pereira FS, Vargas CR, Coelho DM, Torrez V, Oses JP, Portela LV, Schestatsky P, Felix VT, Matte U, Torman VL, Jardim LB | title = Progression rate of myelopathy in X-linked adrenoleukodystrophy heterozygotes | journal = Metabolic Brain Disease | volume = 30 | issue = 5 | pages = 1279–84 | date = October 2015 | pmid = 25920484 | doi = 10.1007/s11011-015-9672-2 }}</ref> |
||
The differentiation of phenotype in heterozygous females is furthered by the presence of X-inactivation skewing. Typically, each X-chromosome is silenced in half of the cells, but this process is skewed when preferential inactivation of a chromosome occurs. It is thought that skewing happens either by chance or by a physical characteristic of a chromosome that may cause it to be silenced more or less often, such as an unfavorable mutation.<ref name=":0">{{cite journal | vauthors = Belmont JW |
The differentiation of phenotype in heterozygous females is furthered by the presence of X-inactivation skewing. Typically, each X-chromosome is silenced in half of the cells, but this process is skewed when preferential inactivation of a chromosome occurs. It is thought that skewing happens either by chance or by a physical characteristic of a chromosome that may cause it to be silenced more or less often, such as an unfavorable mutation.<ref name=":0">{{cite journal | vauthors = Belmont JW | title = Genetic control of X inactivation and processes leading to X-inactivation skewing | journal = American Journal of Human Genetics | volume = 58 | issue = 6 | pages = 1101–8 | date = June 1996 | pmid = 8651285 }}</ref><ref name=":1">{{cite journal | vauthors = Holle JR, Marsh RA, Holdcroft AM, Davies SM, Wang L, Zhang K, Jordan MB | title = Hemophagocytic lymphohistiocytosis in a female patient due to a heterozygous XIAP mutation and skewed X chromosome inactivation | journal = Pediatric Blood & Cancer | volume = 62 | issue = 7 | pages = 1288–90 | date = July 2015 | pmid = 25801017 | doi = 10.1002/pbc.25483 }}</ref> |
||
On average, each X chromosome is inactivated in half of the cells, however 5-20% of "apparently normal" women display X-inactivation skewing.<ref name=":0" /> In cases where skewing is present, a broad range of symptom expression can occur, resulting in expression varying from minor to severe depending on the skewing proportion. An extreme case of this was seen where monozygotic female twins had extreme variance in expression of Menkes disease (an X-linked disorder) resulting in the death of one twin while the other remained asymptomatic.<ref>{{cite journal | |
On average, each X chromosome is inactivated in half of the cells, however 5-20% of "apparently normal" women display X-inactivation skewing.<ref name=":0" /> In cases where skewing is present, a broad range of symptom expression can occur, resulting in expression varying from minor to severe depending on the skewing proportion. An extreme case of this was seen where monozygotic female twins had extreme variance in expression of Menkes disease (an X-linked disorder) resulting in the death of one twin while the other remained asymptomatic.<ref>{{cite journal | vauthors = Burgemeister AL, Zirn B, Oeffner F, Kaler SG, Lemm G, Rossier E, Büttel HM | title = Menkes disease with discordant phenotype in female monozygotic twins | journal = American Journal of Medical Genetics. Part A | volume = 167A | issue = 11 | pages = 2826–9 | date = November 2015 | pmid = 26239182 | doi = 10.1002/ajmg.a.37276 }}</ref> |
||
It’s thought that X-inactivation skewing could be caused by issues in the mechanism that causes inactivation, or by issues in the chromosome itself.<ref name=":0" /><ref name=":1" /> However, the link between phenotype and skewing is still being questioned, and should be examined on a case-by-case basis. A study looking at both symptomatic and asymptomatic females who were heterozygous for [[Duchenne muscular dystrophy|Duchenne]] and Becker muscular dystrophies (DMD) found no apparent link between transcript expression and skewed X-Inactivation. The study suggests that both mechanisms are independently regulated, and there are other unknown factors at play.<ref>Brioschi |
It’s thought that X-inactivation skewing could be caused by issues in the mechanism that causes inactivation, or by issues in the chromosome itself.<ref name=":0" /><ref name=":1" /> However, the link between phenotype and skewing is still being questioned, and should be examined on a case-by-case basis. A study looking at both symptomatic and asymptomatic females who were heterozygous for [[Duchenne muscular dystrophy|Duchenne]] and Becker muscular dystrophies (DMD) found no apparent link between transcript expression and skewed X-Inactivation. The study suggests that both mechanisms are independently regulated, and there are other unknown factors at play.<ref name="pmid22894145">{{cite journal | vauthors = Brioschi S, Gualandi F, Scotton C, Armaroli A, Bovolenta M, Falzarano MS, Sabatelli P, Selvatici R, D'Amico A, Pane M, Ricci G, Siciliano G, Tedeschi S, Pini A, Vercelli L, De Grandis D, Mercuri E, Bertini E, Merlini L, Mongini T, Ferlini A | title = Genetic characterization in symptomatic female DMD carriers: lack of relationship between X-inactivation, transcriptional DMD allele balancing and phenotype | journal = BMC Medical Genetics | volume = 13 | issue = | pages = 73 | date = August 2012 | pmid = 22894145 | pmc = 3459813 | doi = 10.1186/1471-2350-13-73 }}</ref> |
||
===Chromosomal component=== |
===Chromosomal component=== |
||
| Line 98: | Line 98: | ||
===Xist and Tsix RNAs=== |
===Xist and Tsix RNAs=== |
||
{{Main|Xist}} |
{{Main|Xist}} |
||
The X-inactive specific transcript ([[Xist]]) gene encodes a large [[NcRNA|non-coding RNA]] that is responsible for mediating the specific silencing of the X chromosome from which it is transcribed.<ref>{{cite journal |vauthors=Hoki Y, Kimura N, Kanbayashi M, Amakawa Y, Ohhata T, Sasaki H, Sado T | |
The X-inactive specific transcript ([[Xist]]) gene encodes a large [[NcRNA|non-coding RNA]] that is responsible for mediating the specific silencing of the X chromosome from which it is transcribed.<ref>{{cite journal | vauthors = Hoki Y, Kimura N, Kanbayashi M, Amakawa Y, Ohhata T, Sasaki H, Sado T | title = A proximal conserved repeat in the Xist gene is essential as a genomic element for X-inactivation in mouse | journal = Development | volume = 136 | issue = 1 | pages = 139–46 | date = January 2009 | pmid = 19036803 | doi = 10.1242/dev.026427 }}</ref> The inactive X chromosome is coated by Xist RNA,<ref>{{cite journal | vauthors = Ng K, Pullirsch D, Leeb M, Wutz A | title = Xist and the order of silencing | journal = EMBO Reports | volume = 8 | issue = 1 | pages = 34–9 | date = January 2007 | pmid = 17203100 | pmc = 1796754 | doi = 10.1038/sj.embor.7400871 | format = Review Article | quote = [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1796754&rendertype=figure&id=f1 Figure 1 Xist RNA encompasses the X from which it is transcribed.] }}</ref> whereas the Xa is not (See Figure to the right). X chromosomes that lack the Xist gene cannot be inactivated.<ref>{{cite journal | vauthors = Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N | title = Requirement for Xist in X chromosome inactivation | journal = Nature | volume = 379 | issue = 6561 | pages = 131–7 | date = January 1996 | pmid = 8538762 | doi = 10.1038/379131a0 }}</ref> Artificially placing and expressing the Xist gene on another chromosome leads to silencing of that chromosome.<ref name="Herzing">{{cite journal | vauthors = Herzing LB, Romer JT, Horn JM, Ashworth A | title = Xist has properties of the X-chromosome inactivation centre | journal = Nature | volume = 386 | issue = 6622 | pages = 272–5 | date = March 1997 | pmid = 9069284 | doi = 10.1038/386272a0 }}</ref><ref>{{cite journal | vauthors = Lee JT, Jaenisch R | title = Long-range cis effects of ectopic X-inactivation centres on a mouse autosome | journal = Nature | volume = 386 | issue = 6622 | pages = 275–9 | date = March 1997 | pmid = 9069285 | doi = 10.1038/386275a0 }}</ref> |
||
Prior to inactivation, both X chromosomes weakly express Xist RNA from the Xist gene. During the inactivation process, the future Xa ceases to express Xist, whereas the future Xi dramatically increases Xist RNA production. On the future Xi, the Xist RNA progressively coats the chromosome, spreading out from the XIC;<ref name=Herzing/> the Xist RNA does not localize to the Xa. The [[gene silencing|silencing of genes]] along the Xi occurs soon after coating by Xist RNA. |
Prior to inactivation, both X chromosomes weakly express Xist RNA from the Xist gene. During the inactivation process, the future Xa ceases to express Xist, whereas the future Xi dramatically increases Xist RNA production. On the future Xi, the Xist RNA progressively coats the chromosome, spreading out from the XIC;<ref name=Herzing/> the Xist RNA does not localize to the Xa. The [[gene silencing|silencing of genes]] along the Xi occurs soon after coating by Xist RNA. |
||
Like Xist, the [[Tsix]] gene encodes a large RNA which is not believed to encode a protein. The Tsix RNA is transcribed [[antisense]] to Xist, meaning that the Tsix gene overlaps the Xist gene and is [[transcription (genetics)|transcribed]] on the opposite strand of [[DNA]] from the Xist gene.<ref>{{cite journal |vauthors=Lee JT, Davidow LS, Warshawsky D | |
Like Xist, the [[Tsix]] gene encodes a large RNA which is not believed to encode a protein. The Tsix RNA is transcribed [[antisense]] to Xist, meaning that the Tsix gene overlaps the Xist gene and is [[transcription (genetics)|transcribed]] on the opposite strand of [[DNA]] from the Xist gene.<ref>{{cite journal | vauthors = Lee JT, Davidow LS, Warshawsky D | title = Tsix, a gene antisense to Xist at the X-inactivation centre | journal = Nature Genetics | volume = 21 | issue = 4 | pages = 400–4 | date = April 1999 | pmid = 10192391 | doi = 10.1038/7734 }}</ref> Tsix is a negative regulator of Xist; X chromosomes lacking Tsix expression (and thus having high levels of Xist transcription) are inactivated much more frequently than normal chromosomes. |
||
Like Xist, prior to inactivation, both X chromosomes weakly express Tsix RNA from the Tsix gene. Upon the onset of X-inactivation, the future Xi ceases to express Tsix RNA (and increases Xist expression), whereas Xa continues to express Tsix for several days. |
Like Xist, prior to inactivation, both X chromosomes weakly express Tsix RNA from the Tsix gene. Upon the onset of X-inactivation, the future Xi ceases to express Tsix RNA (and increases Xist expression), whereas Xa continues to express Tsix for several days. |
||
| Line 111: | Line 111: | ||
The inactive X chromosome does not express the majority of its genes, unlike the active X chromosome. This is due to the silencing of the Xi by repressive [[heterochromatin]], which compacts the Xi DNA and prevents the expression of most genes. |
The inactive X chromosome does not express the majority of its genes, unlike the active X chromosome. This is due to the silencing of the Xi by repressive [[heterochromatin]], which compacts the Xi DNA and prevents the expression of most genes. |
||
Compared to the Xa, the Xi has high levels of [[DNA methylation]], low levels of [[histone acetylation]], low levels of [[histone H3]] lysine-4 [[histone methylation|methylation]], and high levels of histone H3 lysine-9 methylation and H3 lysine-27 methylation mark which is placed by the [[Polycomb recruitment in X chromosome inactivation|PRC2 complex recruited by Xist]], all of which are associated with gene silencing.<ref>{{cite journal |vauthors=Ng K, Pullirsch D, Leeb M, Wutz A | |
Compared to the Xa, the Xi has high levels of [[DNA methylation]], low levels of [[histone acetylation]], low levels of [[histone H3]] lysine-4 [[histone methylation|methylation]], and high levels of histone H3 lysine-9 methylation and H3 lysine-27 methylation mark which is placed by the [[Polycomb recruitment in X chromosome inactivation|PRC2 complex recruited by Xist]], all of which are associated with gene silencing.<ref>{{cite journal | vauthors = Ng K, Pullirsch D, Leeb M, Wutz A | title = Xist and the order of silencing | journal = EMBO Reports | volume = 8 | issue = 1 | pages = 34–9 | date = January 2007 | pmid = 17203100 | pmc = 1796754 | doi = 10.1038/sj.embor.7400871 | format = Review Article | quote = [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1796754&rendertype=table&id=t1 Table 1 Features of the inactive X territory] }} – Originated from;<br/>{{cite journal | vauthors = Chow JC, Yen Z, Ziesche SM, Brown CJ | title = Silencing of the mammalian X chromosome | journal = Annual Review of Genomics and Human Genetics | volume = 6 | pages = 69–92 | year = 2005 | pmid = 16124854 | doi = 10.1146/annurev.genom.6.080604.162350 }}<br/>{{cite journal | vauthors = Lucchesi JC, Kelly WG, Panning B | title = Chromatin remodeling in dosage compensation | journal = Annual Review of Genetics | volume = 39 | pages = 615–51 | year = 2005 | pmid = 16285873 | doi = 10.1146/annurev.genet.39.073003.094210 }}</ref> Additionally, a histone variant called macroH2A ([[H2AFY]]) is exclusively found on [[nucleosome]]s along the Xi.<ref name="Costanzi1998">{{cite journal | vauthors = Costanzi C, Pehrson JR | title = Histone macroH2A1 is concentrated in the inactive X chromosome of female mammals | journal = Nature | volume = 393 | issue = 6685 | pages = 599–601 | date = June 1998 | pmid = 9634239 | doi = 10.1038/31275 }}</ref><ref name="Costanzi2000">{{cite journal | vauthors = Costanzi C, Stein P, Worrad DM, Schultz RM, Pehrson JR | title = Histone macroH2A1 is concentrated in the inactive X chromosome of female preimplantation mouse embryos | journal = Development | volume = 127 | issue = 11 | pages = 2283–9 | date = June 2000 | pmid = 10804171 | url = http://dev.biologists.org/cgi/reprint/127/11/2283.pdf | format = pdf }}</ref> |
||
===Barr bodies=== |
===Barr bodies=== |
||
{{main|Barr body}} |
{{main|Barr body}} |
||
DNA packaged in heterochromatin, such as the Xi, is more condensed than DNA packaged in [[euchromatin]], such as the Xa. The inactive X forms a discrete body within the nucleus called a [[Barr body]].<ref name="barr 1949">{{cite journal |vauthors=Barr ML, Bertram EG | |
DNA packaged in heterochromatin, such as the Xi, is more condensed than DNA packaged in [[euchromatin]], such as the Xa. The inactive X forms a discrete body within the nucleus called a [[Barr body]].<ref name="barr 1949">{{cite journal | vauthors = Barr ML, Bertram EG | title = A morphological distinction between neurones of the male and female, and the behaviour of the nucleolar satellite during accelerated nucleoprotein synthesis | journal = Nature | volume = 163 | issue = 4148 | pages = 676 | date = April 1949 | pmid = 18120749 | doi = 10.1038/163676a0 }}</ref> The Barr body is generally located on the periphery of the [[cell nucleus|nucleus]], is late [[DNA replication|replicating]] within the [[cell cycle]], and, as it contains the Xi, contains heterochromatin modifications and the Xist RNA. |
||
====Expressed genes on the inactive X chromosome==== |
====Expressed genes on the inactive X chromosome==== |
||
A fraction of the genes along the X chromosome escape inactivation on the Xi. The Xist gene is expressed at high levels on the Xi and is not expressed on the Xa.<ref name="Plath">{{cite journal |vauthors=Plath K, Mlynarczyk-Evans S, Nusinow |
A fraction of the genes along the X chromosome escape inactivation on the Xi. The Xist gene is expressed at high levels on the Xi and is not expressed on the Xa.<ref name="Plath">{{cite journal | vauthors = Plath K, Mlynarczyk-Evans S, Nusinow DA, Panning B | title = Xist RNA and the mechanism of X chromosome inactivation | journal = Annual Review of Genetics | volume = 36 | pages = 233–78 | year = 2002 | pmid = 12429693 | doi = 10.1146/annurev.genet.36.042902.092433 }}</ref> Many other genes escape inactivation; some are expressed equally from the Xa and Xi, and others, while expressed from both chromosomes, are still predominantly expressed from the Xa.<ref name="Carrel L, Willard H 2005 400–404">{{cite journal | vauthors = Carrel L, Willard HF | title = X-inactivation profile reveals extensive variability in X-linked gene expression in females | journal = Nature | volume = 434 | issue = 7031 | pages = 400–4 | date = March 2005 | pmid = 15772666 | doi = 10.1038/nature03479 }}</ref><ref name="Calabrese JM, Sun W, Song L, Mugford JW, Williams L, Yee D, Starmer J, Mieczkowski P, Crawford GE, Magnuson T 2012 951–63">{{cite journal | vauthors = Calabrese JM, Sun W, Song L, Mugford JW, Williams L, Yee D, Starmer J, Mieczkowski P, Crawford GE, Magnuson T | title = Site-specific silencing of regulatory elements as a mechanism of X inactivation | journal = Cell | volume = 151 | issue = 5 | pages = 951–63 | date = November 2012 | pmid = 23178118 | pmc = 3511858 | doi = 10.1016/j.cell.2012.10.037 }}</ref><ref name="Yang F, Babak T, Shendure J, Disteche CM 2010 614–22">{{cite journal | vauthors = Yang F, Babak T, Shendure J, Disteche CM | title = Global survey of escape from X inactivation by RNA-sequencing in mouse | journal = Genome Research | volume = 20 | issue = 5 | pages = 614–22 | date = May 2010 | pmid = 20363980 | pmc = 2860163 | doi = 10.1101/gr.103200.109 }}</ref> Up to one quarter of genes on the human Xi are capable of escape.<ref name="Carrel L, Willard H 2005 400–404"/> Studies in the mouse suggest that in any given cell type, 3% to 15% of genes escape inactivation, and that escaping gene identity varies between tissues.<ref name="Calabrese JM, Sun W, Song L, Mugford JW, Williams L, Yee D, Starmer J, Mieczkowski P, Crawford GE, Magnuson T 2012 951–63"/><ref name="Yang F, Babak T, Shendure J, Disteche CM 2010 614–22"/> |
||
Many of the genes which escape inactivation are present along regions of the X chromosome which, unlike the majority of the X chromosome, contain genes also present on the [[Y chromosome]]. These regions are termed [[pseudoautosomal]] regions, as individuals of either sex will receive two copies of every gene in these regions (like an autosome), unlike the majority of genes along the sex chromosomes. Since individuals of either sex will receive two copies of every gene in a [[pseudoautosomal region]], no dosage compensation is needed for females, so it is postulated that these regions of DNA have evolved mechanisms to escape X-inactivation. The genes of pseudoautosomal regions of the Xi do not have the typical modifications of the Xi and have little Xist RNA bound. |
Many of the genes which escape inactivation are present along regions of the X chromosome which, unlike the majority of the X chromosome, contain genes also present on the [[Y chromosome]]. These regions are termed [[pseudoautosomal]] regions, as individuals of either sex will receive two copies of every gene in these regions (like an autosome), unlike the majority of genes along the sex chromosomes. Since individuals of either sex will receive two copies of every gene in a [[pseudoautosomal region]], no dosage compensation is needed for females, so it is postulated that these regions of DNA have evolved mechanisms to escape X-inactivation. The genes of pseudoautosomal regions of the Xi do not have the typical modifications of the Xi and have little Xist RNA bound. |
||
| Line 125: | Line 125: | ||
The existence of genes along the inactive X which are not silenced explains the defects in humans with abnormal numbers of the X chromosome, such as [[Turner syndrome]] (X0) or [[Klinefelter syndrome]] (XXY). Theoretically, X-inactivation should eliminate the differences in gene dosage between affected individuals and individuals with a normal chromosome complement. In affected individuals, however, X-inactivation is incomplete and the dosage of these non-silenced genes will differ as they escape X-inactivation, similar to an autosomal [[aneuploidy]]. |
The existence of genes along the inactive X which are not silenced explains the defects in humans with abnormal numbers of the X chromosome, such as [[Turner syndrome]] (X0) or [[Klinefelter syndrome]] (XXY). Theoretically, X-inactivation should eliminate the differences in gene dosage between affected individuals and individuals with a normal chromosome complement. In affected individuals, however, X-inactivation is incomplete and the dosage of these non-silenced genes will differ as they escape X-inactivation, similar to an autosomal [[aneuploidy]]. |
||
The precise mechanisms that control escape from X-inactivation are not known, but silenced and escape regions have been shown to have distinct chromatin marks.<ref name="Calabrese JM, Sun W, Song L, Mugford JW, Williams L, Yee D, Starmer J, Mieczkowski P, Crawford GE, Magnuson T 2012 951–63"/><ref>{{cite journal |vauthors=Berletch JB, Yang F, Disteche CM |title=Escape from X inactivation in mice and humans |journal=Genome Biology |volume=11 |issue=6 |pages=213 |date=June 2010 |pmid=20573260 |pmc=2911101 |doi=10.1186/gb-2010-11-6-213 }}</ref> It has been suggested that escape from X-inactivation might be mediated by expression of [[long non-coding RNA]] (lncRNA) within the escaping chromosomal domains.<ref name="pmid21047393">{{cite journal | |
The precise mechanisms that control escape from X-inactivation are not known, but silenced and escape regions have been shown to have distinct chromatin marks.<ref name="Calabrese JM, Sun W, Song L, Mugford JW, Williams L, Yee D, Starmer J, Mieczkowski P, Crawford GE, Magnuson T 2012 951–63"/><ref>{{cite journal | vauthors = Berletch JB, Yang F, Disteche CM | title = Escape from X inactivation in mice and humans | journal = Genome Biology | volume = 11 | issue = 6 | pages = 213 | date = June 2010 | pmid = 20573260 | pmc = 2911101 | doi = 10.1186/gb-2010-11-6-213 }}</ref> It has been suggested that escape from X-inactivation might be mediated by expression of [[long non-coding RNA]] (lncRNA) within the escaping chromosomal domains.<ref name="pmid21047393">{{cite journal | vauthors = Reinius B, Shi C, Hengshuo L, Sandhu KS, Radomska KJ, Rosen GD, Lu L, Kullander K, Williams RW, Jazin E | title = Female-biased expression of long non-coding RNAs in domains that escape X-inactivation in mouse | journal = BMC Genomics | volume = 11 | pages = 614 | date = November 2010 | pmid = 21047393 | pmc = 3091755 | doi = 10.1186/1471-2164-11-614 }}</ref> |
||
==Uses in experimental biology== |
==Uses in experimental biology== |
||
[[Stanley Michael Gartler]] used X chromosome inactivation to demonstrate the clonal origin of cancers. Examining normal tissues and tumors from females heterozygous for isoenzymes of the sex-linked [[glucose-6-phosphate dehydrogenase|G6PD]] gene demonstrated that tumor cells from such individuals express only one form of G6PD, whereas normal tissues are composed of a nearly equal mixture of cells expressing the two different phenotypes. This pattern suggests that a single cell, and not a population, grows into a cancer.<ref>{{cite journal |vauthors=Linder D, Gartler SM |title=Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas |journal=Science |volume=150 |issue=3692 |pages=67–9 |date=October 1965 |pmid=5833538 |doi=10.1126/science.150.3692.67 }}</ref> However, this pattern has been proven wrong for many cancer types, suggesting that some cancers may be polyclonal in origin.<ref>{{cite journal | |
[[Stanley Michael Gartler]] used X chromosome inactivation to demonstrate the clonal origin of cancers. Examining normal tissues and tumors from females heterozygous for isoenzymes of the sex-linked [[glucose-6-phosphate dehydrogenase|G6PD]] gene demonstrated that tumor cells from such individuals express only one form of G6PD, whereas normal tissues are composed of a nearly equal mixture of cells expressing the two different phenotypes. This pattern suggests that a single cell, and not a population, grows into a cancer.<ref>{{cite journal | vauthors = Linder D, Gartler SM | title = Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas | journal = Science | volume = 150 | issue = 3692 | pages = 67–9 | date = October 1965 | pmid = 5833538 | doi = 10.1126/science.150.3692.67 }}</ref> However, this pattern has been proven wrong for many cancer types, suggesting that some cancers may be polyclonal in origin.<ref>{{cite journal | vauthors = Parsons BL | title = Many different tumor types have polyclonal tumor origin: evidence and implications | journal = Mutation Research | volume = 659 | issue = 3 | pages = 232–47 | year = 2008 | pmid = 18614394 | doi = 10.1016/j.mrrev.2008.05.004 }}</ref> |
||
Besides, measuring the methylation (inactivation) status of the polymorphic human androgen receptor (HUMARA) located on X-chromosome is considered the most accurate method to assess clonality in female cancer biopsies.<ref>{{cite journal | |
Besides, measuring the methylation (inactivation) status of the polymorphic human androgen receptor (HUMARA) located on X-chromosome is considered the most accurate method to assess clonality in female cancer biopsies.<ref>{{cite journal | vauthors = Chen GL, Prchal JT | title = X-linked clonality testing: interpretation and limitations | journal = Blood | volume = 110 | issue = 5 | pages = 1411–9 | date = September 2007 | pmid = 17435115 | pmc = 1975831 | doi = 10.1182/blood-2006-09-018655 }}</ref> A great variety of tumors was tested by this method, some, such as renal cell carcinoma,<ref>{{cite journal | vauthors = Petersson F, Branzovsky J, Martinek P, Korabecna M, Kruslin B, Hora M, Peckova K, Bauleth K, Pivovarcikova K, Michal M, Svajdler M, Sperga M, Bulimbasic S, Leroy X, Rychly B, Trivunic S, Kokoskova B, Rotterova P, Podhola M, Suster S, Hes O | title = The leiomyomatous stroma in renal cell carcinomas is polyclonal and not part of the neoplastic process | journal = Virchows Archiv | volume = 465 | issue = 1 | pages = 89–96 | date = July 2014 | pmid = 24838683 | doi = 10.1007/s00428-014-1591-9 }}</ref> found monoclonal while others (e.g. mesothelioma<ref>{{cite journal | vauthors = Comertpay S, Pastorino S, Tanji M, Mezzapelle R, Strianese O, Napolitano A, Baumann F, Weigel T, Friedberg J, Sugarbaker P, Krausz T, Wang E, Powers A, Gaudino G, Kanodia S, Pass HI, Parsons BL, Yang H, Carbone M | title = Evaluation of clonal origin of malignant mesothelioma | journal = Journal of Translational Medicine | volume = 12 | issue = | pages = 301 | date = December 2014 | pmid = 25471750 | pmc = 4255423 | doi = 10.1186/s12967-014-0301-3 }}</ref>) were reported polyclonal. |
||
Researchers have also investigated using X-chromosome inactivation to silence the activity of autosomal chromosomes. For example, Jiang ''et al.'' inserted a copy of the Xist gene into one copy of chromosome 21 in [[IPS cell|stem cells]] derived from an individual with trisomy 21 ([[Down's syndrome]]).<ref>{{cite journal | |
Researchers have also investigated using X-chromosome inactivation to silence the activity of autosomal chromosomes. For example, Jiang ''et al.'' inserted a copy of the Xist gene into one copy of chromosome 21 in [[IPS cell|stem cells]] derived from an individual with trisomy 21 ([[Down's syndrome]]).<ref>{{cite journal | vauthors = Jiang J, Jing Y, Cost GJ, Chiang JC, Kolpa HJ, Cotton AM, Carone DM, Carone BR, Shivak DA, Guschin DY, Pearl JR, Rebar EJ, Byron M, Gregory PD, Brown CJ, Urnov FD, Hall LL, Lawrence JB | title = Translating dosage compensation to trisomy 21 | journal = Nature | volume = 500 | issue = 7462 | pages = 296–300 | date = August 2013 | pmid = 23863942 | pmc = 3848249 | doi = 10.1038/nature12394 }}</ref> The inserted Xist gene induces Barr body formation, triggers stable heterochromatin modifications, and silences most of the genes on the extra copy of chromosome 21. In these modified stem cells, the Xist-mediated gene silencing seems to reverse some of the defects associated with Down's syndrome. |
||
==See also== |
== See also == |
||
*[[Sex-determination system]] |
*[[Sex-determination system]] |
||
*[[Dosage compensation]] |
*[[Dosage compensation]] |
||
| Line 145: | Line 145: | ||
**[[Frontonasal dysplasia]] |
**[[Frontonasal dysplasia]] |
||
==References== |
== References == |
||
{{Reflist}} |
{{Reflist}} |
||
*{{cite journal |vauthors=Huynh KD, Lee JT |title=X-chromosome inactivation: a hypothesis linking ontogeny and phylogeny |journal=Nature Reviews Genetics |volume=6 |issue=5 |pages=410–18 |year=2005 |pmid=15818384 |doi=10.1038/nrg1604}} |
|||
* [http://www.abc.net.au/science/k2/moments/s1002754.htm X-inactivation as a possible cause for autoimmunity] |
|||
== Further reading == |
== Further reading == |
||
{{refbegin}} |
|||
Review Article |
|||
* {{cite journal |vauthors= |
* {{cite journal | vauthors = Huynh KD, Lee JT | title = X-chromosome inactivation: a hypothesis linking ontogeny and phylogeny | journal = Nature Reviews. Genetics | volume = 6 | issue = 5 | pages = 410–8 | date = May 2005 | pmid = 15818384 | doi = 10.1038/nrg1604 }} |
||
* {{cite journal | |
* {{cite journal | vauthors = Goto T, Monk M | title = Regulation of X-chromosome inactivation in development in mice and humans | journal = Microbiology and Molecular Biology Reviews | volume = 62 | issue = 2 | pages = 362–78 | date = June 1998 | pmid = 9618446 | pmc = 98919 | format = Review Article }} |
||
* {{cite journal |vauthors= |
* {{cite journal | vauthors = Lyon MF | title = The Lyon and the LINE hypothesis | journal = Seminars in Cell & Developmental Biology | volume = 14 | issue = 6 | pages = 313–8 | date = December 2003 | pmid = 15015738 | doi = 10.1016/j.semcdb.2003.09.015 | format = Review Article }} |
||
* {{cite journal |vauthors= |
* {{cite journal | vauthors = Ng K, Pullirsch D, Leeb M, Wutz A | title = Xist and the order of silencing | journal = EMBO Reports | volume = 8 | issue = 1 | pages = 34–9 | date = January 2007 | pmid = 17203100 | pmc = 1796754 | doi = 10.1038/sj.embor.7400871 | format = Review Article }} |
||
* {{cite journal | vauthors = Cerase A, Pintacuda G, Tattermusch A, Avner P | title = Xist localization and function: new insights from multiple levels | journal = Genome Biology | volume = 16 | issue = | pages = 166 | date = August 2015 | pmid = 26282267 | pmc = 4539689 | doi = 10.1186/s13059-015-0733-y }} |
|||
{{refend}} |
|||
== External links == |
|||
* {{cite web | first = Karl | last = Kruszelnicki | name-list-format = vanc | url = http://www.abc.net.au/science/k2/moments/s1002754.htm | title = Hybrid Auto-Immune Women 3 | work = ABC Science }} |
|||
{{Commons category|X chromosome inactivation}} |
{{Commons category|X chromosome inactivation}} |
||
Revision as of 03:23, 20 May 2018


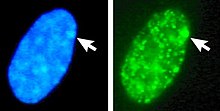
Left: DNA (DAPI)-stained nucleus. Arrow indicates the location of Barr body(Xi). Right: DNA associated histones protein detected

X-inactivation (also called lyonization) is a process by which one of the copies of the X chromosome present in female mammals is inactivated. The inactive X chromosome is silenced by it being packaged in such a way that it has a transcriptionally inactive structure called heterochromatin. As nearly all female mammals have two X chromosomes, X-inactivation prevents them from having twice as many X chromosome gene products as males, who only possess a single copy of the X chromosome (see dosage compensation). The choice of which X chromosome will be inactivated is random in placental mammals such as humans, but once an X chromosome is inactivated it will remain inactive throughout the lifetime of the cell and its descendants in the organism. Unlike the random X-inactivation in placental mammals, inactivation in marsupials applies exclusively to the paternally derived X chromosome.
History
In 1959 Susumu Ohno showed that the two X-chromosomes of mammals were different: one appeared similar to the autosomes; the other was condensed and heterochromatic.[3] This finding suggested, independently to two groups of investigators, that one of the X-chromosomes underwent inactivation. In 1961, Mary Lyon proposed the random inactivation of one female X chromosome to explain the mottled phenotype of female mice heterozygous for coat color genes.[4] The Lyon hypothesis also accounted for the findings that one copy of the X chromosome in female cells was highly condensed, and that mice with only one copy of the X chromosome developed as infertile females. This suggested[5] to Ernest Beutler, studying heterozygous females for Glucose-6-phosphate dehydrogenase (G6PD) deficiency, that there were two red cell populations of erythrocytes in such heterozygotes: deficient cells and normal cells,[6] depending on whether the inactivated X chromosome (in the nucleus of the red cell's precursor cell) contains the normal or defective G6PD allele.
Mechanism
Cycle of X chromosome activation
X-inactivation is part of the activation cycle of the X chromosome throughout the female life. The egg, and the fertilized zygote initially uses maternal transcripts, and the whole embryonic genome is silenced, which lasts until lasting until zygotic genome activation. Thereafter, all mouse cells undergo an early, imprinted inactivation of the paternally-derived X chromosome in 4-8 cell stage embryos [7][8][9][10][11]. The extraembryonic tissues (which give rise to the placenta and other tissues supporting the embryo) retain this early imprinted inactivation, and thus only the maternal X chromosome is active in these tissues.
In the early blastocyst, this initial, imprinted X-inactivation is reversed in the cells of the inner cell mass (which give rise to the embryo), and in these cells both X chromosomes become active again. Each of these cells then independently and randomly inactivates one copy of the X chromosome.[9] This inactivation event is irreversible during the lifetime of the individual, with the exception of the germline. In the female germline before meiotic entry, X-inactivation is reversed, so that after meiosis all haploid oocytes contain an active X chromosome.
Overview
The Xi marks the inactive, Xa the active X chromosome. XP denotes the paternal, and XM to denotes the maternal X chromosome. When the egg (carrying XM), is fertilized by a sperm (carrying a Y or an XP) a diploid zygote forms. From zygote, through adult stage, to the next generation of eggs, the X chromosome undergoes the following changes:
- XiP XiM zygote → undergoing zygotic genome activation, leading to:
- XaP XaM → undergoing imprinted (paternal) X-inactivation, leading to:
- XiP XaM → undergoing X-activation in the early blastocyst stage, leading to:
- XaP XaM → undergoing random X-inactivation in the embryonic lineage (inner cell mass) in the blastocyst stage, leading to:
- XiP XaM OR XaP XiM → undergoing X-reactivation in primordial germ cells before meiosis, leading to:
- XaM XaP diploid germ cells in meiotic arrest. As the meiosis I only completes with ovulation, human germ cells exist in this stage from the first weeks of development until puberty. The completion of meiosis leads to:
- XaM AND XaP haploid germ cells (eggs).
The X activation cycle is best studies in mouse, but there are multiple studies in humans. As most of the evidence is coming from mouse, the above scheme represents the events in mice. The completion of the meiosis is simplified here for clarity. Steps 1-4 can be studied in in vitro fertilized embryos, and in differentiating stem cells; X-reactivation happens in the developing embryo, and subsequent (6-7) steps inside the female body, therefore much harder to study.
Timing
The timing of each process depends on the species, and in many cases the precise time is actively debated.
| Process | Mouse | Human | |
| 1 | Zygotic genome activation | 2-4 cell stage[12] | 2-8 cell stage[12] |
| 2 | Imprinted (paternal) X-inactivation | 4-8 cell stage[10][11] | Unclear if it takes place in humans[13] |
| 3 | X-activation | Early blastocyst stage | Early blastocyst stage |
| 4 | Random X-inactivation in the embryonic lineage (inner cell mass) | Late blastocyst stage | Late blastocyst stage, after implantation[13] |
| 5 | X-reactivation in primordial germ cells before meiosis | From before developmental week 4 up to week 14[14][15] |
Inheritance of inactivation status across cell generations
The descendants of each cell which inactivated a particular X chromosome will also inactivate that same chromosome. This phenomenon, which can be observed in the coloration of tortoiseshell cats when females are heterozygous for the X-linked gene, should not be confused with mosaicism, which is a term that specifically refers to differences in the genotype of various cell populations in the same individual; X-inactivation, which is an epigenetic change that results in a different phenotype, is not a change at the genotypic level. For an individual cell or lineage the inactivation is therefore skewed or 'non-random', and this can give rise to mild symptoms in female 'carriers' of X-linked genetic disorders.[16]
Selection of one active X chromosome
Normal females possess two X chromosomes, and in any given cell one chromosome will be active (designated as Xa) and one will be inactive (Xi). However, studies of individuals with extra copies of the X chromosome show that in cells with more than two X chromosomes there is still only one Xa, and all the remaining X chromosomes are inactivated. This indicates that the default state of the X chromosome in females is inactivation, but one X chromosome is always selected to remain active.
It is understood that X-chromosome inactivation is a random process, occurring at about the time of gastrulation in the epiblast (cells that will give rise to the embryo). The maternal and paternal X chromosomes have an equal probability of inactivation. This would suggest that women would be expected to suffer from X-linked disorders approximately 50% as often as men (because women have two X chromosomes, while men have only one); however, in actuality, the occurrence of these disorders in females is much lower than that. One explanation for this disparity is that 12–20% [17] of genes on the inactivated X chromosome remain expressed, thus providing women with added protection against defective genes coded by the X-chromosome. Some[who?] suggest that this disparity must be evidence of preferential (non-random) inactivation. Preferential inactivation of the paternal X-chromosome occurs in both marsupials and in cell lineages that form the membranes surrounding the embryo,[18] whereas in placental mammals either the maternally or the paternally derived X-chromosome may be inactivated in different cell lines.[19]
The time period for X-chromosome inactivation explains this disparity. Inactivation occurs in the epiblast during gastrulation, which gives rise to the embryo.[20] Inactivation occurs on a cellular level, resulting in a mosaic expression, in which patches of cells have an inactive maternal X-chromosome, while other patches have an inactive paternal X-chromosome. For example, a female heterozygous for haemophilia (an X-linked disease) would have about half of her liver cells functioning properly, which is typically enough to ensure normal blood clotting.[21][22] Chance could result in significantly more dysfunctional cells; however, such statistical extremes are unlikely. Genetic differences on the chromosome may also render one X-chromosome more likely to undergo inactivation. Also, if one X-chromosome has a mutation hindering its growth or rendering it non viable, cells which randomly inactivated that X will have a selective advantage over cells which randomly inactivated the normal allele. Thus, although inactivation is initially random, cells that inactivate a normal allele (leaving the mutated allele active) will eventually be overgrown and replaced by functionally normal cells in which nearly all have the same X-chromosome activated.[21]
It is hypothesized[by whom?] that there is an autosomally-encoded 'blocking factor' which binds to the X chromosome and prevents its inactivation. The model postulates that there is a limiting blocking factor, so once the available blocking factor molecule binds to one X chromosome the remaining X chromosome(s) are not protected from inactivation. This model is supported by the existence of a single Xa in cells with many X chromosomes and by the existence of two active X chromosomes in cell lines with twice the normal number of autosomes.[23]
Sequences at the X inactivation center (XIC), present on the X chromosome, control the silencing of the X chromosome. The hypothetical blocking factor is predicted to bind to sequences within the XIC.
Expression of X-Linked Disorders in Heterozygous Females
It’s easy to see the effect of female X heterozygosity in localized traits, such as the unique coat pattern of a calico cat. It can be more difficult, however, to fully understand the expression of un-localized traits in these females, such as the expression of disease.
Since males only have one copy of the X chromosome, all expressed X-chromosomal genes (or alleles, in the case of multiple variant forms for a given gene in the population) are located on that copy of the chromosome. Females, however, will primarily express the genes or alleles located on the X-chromosomal copy that remains active. Considering the situation for one gene or multiple genes causing individual differences in a particular phenotype (i.e., causing variation observed in the population for that phenotype), in homozygous females it doesn’t particularly matter which copy of the chromosome is inactivated, as the alleles on both copies are the same. However, in females that are heterozygous at the causal genes, the inactivation of one copy of the chromosome over the other can have a direct impact on their phenotypic value. Because of this phenomenon, there is an observed increase in phenotypic variation in females that are heterozygous at the involved gene or genes than in females that are homozygous at that gene or those genes.[24] There are many different ways in which the phenotypic variation can play out. In many cases, heterozygous females may be asymptomatic or only present minor symptoms of a given disorder, such as with X-linked adrenoleukodystrophy.[25]
The differentiation of phenotype in heterozygous females is furthered by the presence of X-inactivation skewing. Typically, each X-chromosome is silenced in half of the cells, but this process is skewed when preferential inactivation of a chromosome occurs. It is thought that skewing happens either by chance or by a physical characteristic of a chromosome that may cause it to be silenced more or less often, such as an unfavorable mutation.[26][27]
On average, each X chromosome is inactivated in half of the cells, however 5-20% of "apparently normal" women display X-inactivation skewing.[26] In cases where skewing is present, a broad range of symptom expression can occur, resulting in expression varying from minor to severe depending on the skewing proportion. An extreme case of this was seen where monozygotic female twins had extreme variance in expression of Menkes disease (an X-linked disorder) resulting in the death of one twin while the other remained asymptomatic.[28]
It’s thought that X-inactivation skewing could be caused by issues in the mechanism that causes inactivation, or by issues in the chromosome itself.[26][27] However, the link between phenotype and skewing is still being questioned, and should be examined on a case-by-case basis. A study looking at both symptomatic and asymptomatic females who were heterozygous for Duchenne and Becker muscular dystrophies (DMD) found no apparent link between transcript expression and skewed X-Inactivation. The study suggests that both mechanisms are independently regulated, and there are other unknown factors at play.[29]
Chromosomal component
The X-inactivation center (or simply XIC) on the X chromosome is necessary and sufficient to cause X-inactivation. Chromosomal translocations which place the XIC on an autosome lead to inactivation of the autosome, and X chromosomes lacking the XIC are not inactivated.
The XIC contains four non-translated RNA genes, Xist, Tsix, Jpx and Ftx, which are involved in X-inactivation. The XIC also contains binding sites for both known and unknown regulatory proteins.
Xist and Tsix RNAs
The X-inactive specific transcript (Xist) gene encodes a large non-coding RNA that is responsible for mediating the specific silencing of the X chromosome from which it is transcribed.[30] The inactive X chromosome is coated by Xist RNA,[31] whereas the Xa is not (See Figure to the right). X chromosomes that lack the Xist gene cannot be inactivated.[32] Artificially placing and expressing the Xist gene on another chromosome leads to silencing of that chromosome.[33][34]
Prior to inactivation, both X chromosomes weakly express Xist RNA from the Xist gene. During the inactivation process, the future Xa ceases to express Xist, whereas the future Xi dramatically increases Xist RNA production. On the future Xi, the Xist RNA progressively coats the chromosome, spreading out from the XIC;[33] the Xist RNA does not localize to the Xa. The silencing of genes along the Xi occurs soon after coating by Xist RNA.
Like Xist, the Tsix gene encodes a large RNA which is not believed to encode a protein. The Tsix RNA is transcribed antisense to Xist, meaning that the Tsix gene overlaps the Xist gene and is transcribed on the opposite strand of DNA from the Xist gene.[35] Tsix is a negative regulator of Xist; X chromosomes lacking Tsix expression (and thus having high levels of Xist transcription) are inactivated much more frequently than normal chromosomes.
Like Xist, prior to inactivation, both X chromosomes weakly express Tsix RNA from the Tsix gene. Upon the onset of X-inactivation, the future Xi ceases to express Tsix RNA (and increases Xist expression), whereas Xa continues to express Tsix for several days.
Rep A is a long non coding RNA that works with another long non coding RNA, Xist, for X inactivation. Rep A inhibits the function of Tsix, the antisense of Xist, in conjunction with eliminating expression of Xite. It promotes methylation of the Tsix region by attracting PRC2 and thus inactivating one of the X chromosomes.[36]
Silencing
The inactive X chromosome does not express the majority of its genes, unlike the active X chromosome. This is due to the silencing of the Xi by repressive heterochromatin, which compacts the Xi DNA and prevents the expression of most genes.
Compared to the Xa, the Xi has high levels of DNA methylation, low levels of histone acetylation, low levels of histone H3 lysine-4 methylation, and high levels of histone H3 lysine-9 methylation and H3 lysine-27 methylation mark which is placed by the PRC2 complex recruited by Xist, all of which are associated with gene silencing.[37] Additionally, a histone variant called macroH2A (H2AFY) is exclusively found on nucleosomes along the Xi.[38][39]
Barr bodies
DNA packaged in heterochromatin, such as the Xi, is more condensed than DNA packaged in euchromatin, such as the Xa. The inactive X forms a discrete body within the nucleus called a Barr body.[40] The Barr body is generally located on the periphery of the nucleus, is late replicating within the cell cycle, and, as it contains the Xi, contains heterochromatin modifications and the Xist RNA.
Expressed genes on the inactive X chromosome
A fraction of the genes along the X chromosome escape inactivation on the Xi. The Xist gene is expressed at high levels on the Xi and is not expressed on the Xa.[41] Many other genes escape inactivation; some are expressed equally from the Xa and Xi, and others, while expressed from both chromosomes, are still predominantly expressed from the Xa.[42][43][44] Up to one quarter of genes on the human Xi are capable of escape.[42] Studies in the mouse suggest that in any given cell type, 3% to 15% of genes escape inactivation, and that escaping gene identity varies between tissues.[43][44]
Many of the genes which escape inactivation are present along regions of the X chromosome which, unlike the majority of the X chromosome, contain genes also present on the Y chromosome. These regions are termed pseudoautosomal regions, as individuals of either sex will receive two copies of every gene in these regions (like an autosome), unlike the majority of genes along the sex chromosomes. Since individuals of either sex will receive two copies of every gene in a pseudoautosomal region, no dosage compensation is needed for females, so it is postulated that these regions of DNA have evolved mechanisms to escape X-inactivation. The genes of pseudoautosomal regions of the Xi do not have the typical modifications of the Xi and have little Xist RNA bound.
The existence of genes along the inactive X which are not silenced explains the defects in humans with abnormal numbers of the X chromosome, such as Turner syndrome (X0) or Klinefelter syndrome (XXY). Theoretically, X-inactivation should eliminate the differences in gene dosage between affected individuals and individuals with a normal chromosome complement. In affected individuals, however, X-inactivation is incomplete and the dosage of these non-silenced genes will differ as they escape X-inactivation, similar to an autosomal aneuploidy.
The precise mechanisms that control escape from X-inactivation are not known, but silenced and escape regions have been shown to have distinct chromatin marks.[43][45] It has been suggested that escape from X-inactivation might be mediated by expression of long non-coding RNA (lncRNA) within the escaping chromosomal domains.[2]
Uses in experimental biology
Stanley Michael Gartler used X chromosome inactivation to demonstrate the clonal origin of cancers. Examining normal tissues and tumors from females heterozygous for isoenzymes of the sex-linked G6PD gene demonstrated that tumor cells from such individuals express only one form of G6PD, whereas normal tissues are composed of a nearly equal mixture of cells expressing the two different phenotypes. This pattern suggests that a single cell, and not a population, grows into a cancer.[46] However, this pattern has been proven wrong for many cancer types, suggesting that some cancers may be polyclonal in origin.[47]
Besides, measuring the methylation (inactivation) status of the polymorphic human androgen receptor (HUMARA) located on X-chromosome is considered the most accurate method to assess clonality in female cancer biopsies.[48] A great variety of tumors was tested by this method, some, such as renal cell carcinoma,[49] found monoclonal while others (e.g. mesothelioma[50]) were reported polyclonal.
Researchers have also investigated using X-chromosome inactivation to silence the activity of autosomal chromosomes. For example, Jiang et al. inserted a copy of the Xist gene into one copy of chromosome 21 in stem cells derived from an individual with trisomy 21 (Down's syndrome).[51] The inserted Xist gene induces Barr body formation, triggers stable heterochromatin modifications, and silences most of the genes on the extra copy of chromosome 21. In these modified stem cells, the Xist-mediated gene silencing seems to reverse some of the defects associated with Down's syndrome.
See also
- Sex-determination system
- Dosage compensation
- Barr body
- Heterochromatin
- Epigenetics
- Skewed X-inactivation
- Developmental disorders thought to be related to X-inactivation:
References
- ^ Gartler SM, Varadarajan KR, Luo P, Canfield TK, Traynor J, Francke U, Hansen RS (September 2004). "Normal histone modifications on the inactive X chromosome in ICF and Rett syndrome cells: implications for methyl-CpG binding proteins". BMC Biology. 2: 21. doi:10.1186/1741-7007-2-21. PMC 521681. PMID 15377381.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Reinius B, Shi C, Hengshuo L, Sandhu KS, Radomska KJ, Rosen GD, Lu L, Kullander K, Williams RW, Jazin E (November 2010). "Female-biased expression of long non-coding RNAs in domains that escape X-inactivation in mouse". BMC Genomics. 11: 614. doi:10.1186/1471-2164-11-614. PMC 3091755. PMID 21047393.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Ohno S, Kaplan WD, Kinosita R (October 1959). "Formation of the sex chromatin by a single X-chromosome in liver cells of Rattus norvegicus". Experimental Cell Research. 18 (2): 415–8. doi:10.1016/0014-4827(59)90031-X. PMID 14428474.
- ^ Lyon MF (April 1961). "Gene action in the X-chromosome of the mouse (Mus musculus L.)". Nature. 190 (4773): 372–3. doi:10.1038/190372a0. PMID 13764598.
- ^ Beutler E (January 2008). "Glucose-6-phosphate dehydrogenase deficiency: a historical perspective". Blood. 111 (1): 16–24. doi:10.1182/blood-2007-04-077412. PMID 18156501.
- ^ Beutler E, Yeh M, Fairbanks VF (January 1962). "The normal human female as a mosaic of X-chromosome activity: studies using the gene for C-6-PD-deficiency as a marker". Proceedings of the National Academy of Sciences of the United States of America. 48 (1): 9–16. doi:10.1073/pnas.48.1.9. PMC 285481. PMID 13868717.
- ^ Takagi N, Sasaki M (August 1975). "Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse". Nature. 256 (5519): 640–2. doi:10.1038/256640a0. PMID 1152998.
- ^ Cheng MK, Disteche CM (August 2004). "Silence of the fathers: early X inactivation". BioEssays. 26 (8): 821–4. doi:10.1002/bies.20082. PMID 15273983.
- ^ a b Okamoto I, Otte AP, Allis CD, Reinberg D, Heard E (January 2004). "Epigenetic dynamics of imprinted X inactivation during early mouse development". Science. 303 (5658): 644–9. doi:10.1126/science.1092727. PMID 14671313.
- ^ a b Deng Q, Ramsköld D, Reinius B, Sandberg R (January 2014). "Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells". Science. 343 (6167): 193–6. doi:10.1126/science.1245316. PMID 24408435.
- ^ a b Borensztein M, Syx L, Ancelin K, Diabangouaya P, Picard C, Liu T, Liang JB, Vassilev I, Galupa R, Servant N, Barillot E, Surani A, Chen CJ, Heard E (March 2017). "Xist-dependent imprinted X inactivation and the early developmental consequences of its failure". Nature Structural & Molecular Biology. 24 (3): 226–233. doi:10.1038/nsmb.3365. PMC 5337400. PMID 28134930.
- ^ a b Xue Z, Huang K, Cai C, Cai L, Jiang CY, Feng Y, Liu Z, Zeng Q, Cheng L, Sun YE, Liu JY, Horvath S, Fan G (August 2013). "Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing". Nature. 500 (7464): 593–7. doi:10.1038/nature12364. PMC 4950944. PMID 23892778.
- ^ a b Deng X, Berletch JB, Nguyen DK, Disteche CM (June 2014). "X chromosome regulation: diverse patterns in development, tissues and disease". Nature Reviews. Genetics. 15 (6): 367–78. doi:10.1038/nrg3687. PMC 4117651. PMID 24733023.
- ^ Vértesy Á, Arindrarto W, Roost MS, Reinius B, Torrens-Juaneda V, Bialecka M, Moustakas I, Ariyurek Y, Kuijk E, Mei H, Sandberg R, van Oudenaarden A, Chuva de Sousa Lopes SM (May 2018). "Parental haplotype-specific single-cell transcriptomics reveal incomplete epigenetic reprogramming in human female germ cells". Nature Communications. 9 (1): 1873. doi:10.1038/s41467-018-04215-7. PMC 5951918. PMID 29760424.
- ^ Guo F, Yan L, Guo H, Li L, Hu B, Zhao Y, Yong J, Hu Y, Wang X, Wei Y, Wang W, Li R, Yan J, Zhi X, Zhang Y, Jin H, Zhang W, Hou Y, Zhu P, Li J, Zhang L, Liu S, Ren Y, Zhu X, Wen L, Gao YQ, Tang F, Qiao J (June 2015). "The Transcriptome and DNA Methylome Landscapes of Human Primordial Germ Cells". Cell. 161 (6): 1437–52. doi:10.1016/j.cell.2015.05.015. PMID 26046443.
- ^ Puck JM, Willard HF (January 1998). "X inactivation in females with X-linked disease". The New England Journal of Medicine. 338 (5): 325–8. doi:10.1056/NEJM199801293380611. PMID 9445416.
- ^ Balaton BP, Cotton AM, Brown CJ (30 December 2015). "Derivation of consensus inactivation status for X-linked genes from genome-wide studies". Biology of Sex Differences. 6 (35): 35. doi:10.1186/s13293-015-0053-7. PMID 26719789.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Graves JA (1996). "Mammals that break the rules: genetics of marsupials and monotremes". Annual Review of Genetics. 30: 233–60. doi:10.1146/annurev.genet.30.1.233. PMID 8982455.
- ^ Lyon MF (January 1972). "X-chromosome inactivation and developmental patterns in mammals". Biological Reviews of the Cambridge Philosophical Society. 47 (1): 1–35. doi:10.1111/j.1469-185X.1972.tb00969.x. PMID 4554151.
- ^ Migeon, B (2010). "X chromosome inactivation in human cells". The Biomedical & Life Sciences Collection. Henry Stewart Talks, Ltd. pp. 1–54. Retrieved 15 December 2013.
- ^ a b Gartler, Stanley M; Goldman, Michael A (2001). "X-Chromosome Inactivation" (PDF). Encyclopedia of Life Sciences. Nature Publishing Group: 1–2.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Connallon T, Clark AG (April 2013). "Sex-differential selection and the evolution of X inactivation strategies". PLoS Genetics. 9 (4): e1003440. doi:10.1371/journal.pgen.1003440. PMC 3630082. PMID 23637618.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Barakat, Tahsin Stefan; Gribnau, Joost (2010). "X Chromosome Inactivation and Embryonic Stem Cells". In Meshorer, Eran; Plath, Kathrin (eds.). The Cell Biology of Stem Cells. Landes Bioscience and Springer Science+Business Media.
- ^ Ma L, Hoffman G, Keinan A (March 2015). "X-inactivation informs variance-based testing for X-linked association of a quantitative trait". BMC Genomics. 16: 241. doi:10.1186/s12864-015-1463-y. PMC 4381508. PMID 25880738.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Habekost CT, Pereira FS, Vargas CR, Coelho DM, Torrez V, Oses JP, Portela LV, Schestatsky P, Felix VT, Matte U, Torman VL, Jardim LB (October 2015). "Progression rate of myelopathy in X-linked adrenoleukodystrophy heterozygotes". Metabolic Brain Disease. 30 (5): 1279–84. doi:10.1007/s11011-015-9672-2. PMID 25920484.
- ^ a b c Belmont JW (June 1996). "Genetic control of X inactivation and processes leading to X-inactivation skewing". American Journal of Human Genetics. 58 (6): 1101–8. PMID 8651285.
- ^ a b Holle JR, Marsh RA, Holdcroft AM, Davies SM, Wang L, Zhang K, Jordan MB (July 2015). "Hemophagocytic lymphohistiocytosis in a female patient due to a heterozygous XIAP mutation and skewed X chromosome inactivation". Pediatric Blood & Cancer. 62 (7): 1288–90. doi:10.1002/pbc.25483. PMID 25801017.
- ^ Burgemeister AL, Zirn B, Oeffner F, Kaler SG, Lemm G, Rossier E, Büttel HM (November 2015). "Menkes disease with discordant phenotype in female monozygotic twins". American Journal of Medical Genetics. Part A. 167A (11): 2826–9. doi:10.1002/ajmg.a.37276. PMID 26239182.
- ^ Brioschi S, Gualandi F, Scotton C, Armaroli A, Bovolenta M, Falzarano MS, Sabatelli P, Selvatici R, D'Amico A, Pane M, Ricci G, Siciliano G, Tedeschi S, Pini A, Vercelli L, De Grandis D, Mercuri E, Bertini E, Merlini L, Mongini T, Ferlini A (August 2012). "Genetic characterization in symptomatic female DMD carriers: lack of relationship between X-inactivation, transcriptional DMD allele balancing and phenotype". BMC Medical Genetics. 13: 73. doi:10.1186/1471-2350-13-73. PMC 3459813. PMID 22894145.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Hoki Y, Kimura N, Kanbayashi M, Amakawa Y, Ohhata T, Sasaki H, Sado T (January 2009). "A proximal conserved repeat in the Xist gene is essential as a genomic element for X-inactivation in mouse". Development. 136 (1): 139–46. doi:10.1242/dev.026427. PMID 19036803.
- ^ Ng K, Pullirsch D, Leeb M, Wutz A (January 2007). "Xist and the order of silencing" (Review Article). EMBO Reports. 8 (1): 34–9. doi:10.1038/sj.embor.7400871. PMC 1796754. PMID 17203100.
Figure 1 Xist RNA encompasses the X from which it is transcribed.
{{cite journal}}: External link in|quote= - ^ Penny GD, Kay GF, Sheardown SA, Rastan S, Brockdorff N (January 1996). "Requirement for Xist in X chromosome inactivation". Nature. 379 (6561): 131–7. doi:10.1038/379131a0. PMID 8538762.
- ^ a b Herzing LB, Romer JT, Horn JM, Ashworth A (March 1997). "Xist has properties of the X-chromosome inactivation centre". Nature. 386 (6622): 272–5. doi:10.1038/386272a0. PMID 9069284.
- ^ Lee JT, Jaenisch R (March 1997). "Long-range cis effects of ectopic X-inactivation centres on a mouse autosome". Nature. 386 (6622): 275–9. doi:10.1038/386275a0. PMID 9069285.
- ^ Lee JT, Davidow LS, Warshawsky D (April 1999). "Tsix, a gene antisense to Xist at the X-inactivation centre". Nature Genetics. 21 (4): 400–4. doi:10.1038/7734. PMID 10192391.
- ^ Mercer, T.R., Dinger, M.E., Mattick, J.S., (2009). Long non-coding RNAs: insight into functions. Nature Reviews Genetics. (10) 155-159.
- ^ Ng K, Pullirsch D, Leeb M, Wutz A (January 2007). "Xist and the order of silencing" (Review Article). EMBO Reports. 8 (1): 34–9. doi:10.1038/sj.embor.7400871. PMC 1796754. PMID 17203100.
Table 1 Features of the inactive X territory
{{cite journal}}: External link in|quote=
Chow JC, Yen Z, Ziesche SM, Brown CJ (2005). "Silencing of the mammalian X chromosome". Annual Review of Genomics and Human Genetics. 6: 69–92. doi:10.1146/annurev.genom.6.080604.162350. PMID 16124854.
Lucchesi JC, Kelly WG, Panning B (2005). "Chromatin remodeling in dosage compensation". Annual Review of Genetics. 39: 615–51. doi:10.1146/annurev.genet.39.073003.094210. PMID 16285873. - ^ Costanzi C, Pehrson JR (June 1998). "Histone macroH2A1 is concentrated in the inactive X chromosome of female mammals". Nature. 393 (6685): 599–601. doi:10.1038/31275. PMID 9634239.
- ^ Costanzi C, Stein P, Worrad DM, Schultz RM, Pehrson JR (June 2000). "Histone macroH2A1 is concentrated in the inactive X chromosome of female preimplantation mouse embryos" (pdf). Development. 127 (11): 2283–9. PMID 10804171.
- ^ Barr ML, Bertram EG (April 1949). "A morphological distinction between neurones of the male and female, and the behaviour of the nucleolar satellite during accelerated nucleoprotein synthesis". Nature. 163 (4148): 676. doi:10.1038/163676a0. PMID 18120749.
- ^ Plath K, Mlynarczyk-Evans S, Nusinow DA, Panning B (2002). "Xist RNA and the mechanism of X chromosome inactivation". Annual Review of Genetics. 36: 233–78. doi:10.1146/annurev.genet.36.042902.092433. PMID 12429693.
- ^ a b Carrel L, Willard HF (March 2005). "X-inactivation profile reveals extensive variability in X-linked gene expression in females". Nature. 434 (7031): 400–4. doi:10.1038/nature03479. PMID 15772666.
- ^ a b c Calabrese JM, Sun W, Song L, Mugford JW, Williams L, Yee D, Starmer J, Mieczkowski P, Crawford GE, Magnuson T (November 2012). "Site-specific silencing of regulatory elements as a mechanism of X inactivation". Cell. 151 (5): 951–63. doi:10.1016/j.cell.2012.10.037. PMC 3511858. PMID 23178118.
- ^ a b Yang F, Babak T, Shendure J, Disteche CM (May 2010). "Global survey of escape from X inactivation by RNA-sequencing in mouse". Genome Research. 20 (5): 614–22. doi:10.1101/gr.103200.109. PMC 2860163. PMID 20363980.
- ^ Berletch JB, Yang F, Disteche CM (June 2010). "Escape from X inactivation in mice and humans". Genome Biology. 11 (6): 213. doi:10.1186/gb-2010-11-6-213. PMC 2911101. PMID 20573260.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Linder D, Gartler SM (October 1965). "Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas". Science. 150 (3692): 67–9. doi:10.1126/science.150.3692.67. PMID 5833538.
- ^ Parsons BL (2008). "Many different tumor types have polyclonal tumor origin: evidence and implications". Mutation Research. 659 (3): 232–47. doi:10.1016/j.mrrev.2008.05.004. PMID 18614394.
- ^ Chen GL, Prchal JT (September 2007). "X-linked clonality testing: interpretation and limitations". Blood. 110 (5): 1411–9. doi:10.1182/blood-2006-09-018655. PMC 1975831. PMID 17435115.
- ^ Petersson F, Branzovsky J, Martinek P, Korabecna M, Kruslin B, Hora M, Peckova K, Bauleth K, Pivovarcikova K, Michal M, Svajdler M, Sperga M, Bulimbasic S, Leroy X, Rychly B, Trivunic S, Kokoskova B, Rotterova P, Podhola M, Suster S, Hes O (July 2014). "The leiomyomatous stroma in renal cell carcinomas is polyclonal and not part of the neoplastic process". Virchows Archiv. 465 (1): 89–96. doi:10.1007/s00428-014-1591-9. PMID 24838683.
- ^ Comertpay S, Pastorino S, Tanji M, Mezzapelle R, Strianese O, Napolitano A, Baumann F, Weigel T, Friedberg J, Sugarbaker P, Krausz T, Wang E, Powers A, Gaudino G, Kanodia S, Pass HI, Parsons BL, Yang H, Carbone M (December 2014). "Evaluation of clonal origin of malignant mesothelioma". Journal of Translational Medicine. 12: 301. doi:10.1186/s12967-014-0301-3. PMC 4255423. PMID 25471750.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Jiang J, Jing Y, Cost GJ, Chiang JC, Kolpa HJ, Cotton AM, Carone DM, Carone BR, Shivak DA, Guschin DY, Pearl JR, Rebar EJ, Byron M, Gregory PD, Brown CJ, Urnov FD, Hall LL, Lawrence JB (August 2013). "Translating dosage compensation to trisomy 21". Nature. 500 (7462): 296–300. doi:10.1038/nature12394. PMC 3848249. PMID 23863942.
Further reading
- Huynh KD, Lee JT (May 2005). "X-chromosome inactivation: a hypothesis linking ontogeny and phylogeny". Nature Reviews. Genetics. 6 (5): 410–8. doi:10.1038/nrg1604. PMID 15818384.
- Goto T, Monk M (June 1998). "Regulation of X-chromosome inactivation in development in mice and humans" (Review Article). Microbiology and Molecular Biology Reviews. 62 (2): 362–78. PMC 98919. PMID 9618446.
- Lyon MF (December 2003). "The Lyon and the LINE hypothesis". Seminars in Cell & Developmental Biology. 14 (6): 313–8. doi:10.1016/j.semcdb.2003.09.015. PMID 15015738.
{{cite journal}}:|format=requires|url=(help) - Ng K, Pullirsch D, Leeb M, Wutz A (January 2007). "Xist and the order of silencing" (Review Article). EMBO Reports. 8 (1): 34–9. doi:10.1038/sj.embor.7400871. PMC 1796754. PMID 17203100.
- Cerase A, Pintacuda G, Tattermusch A, Avner P (August 2015). "Xist localization and function: new insights from multiple levels". Genome Biology. 16: 166. doi:10.1186/s13059-015-0733-y. PMC 4539689. PMID 26282267.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
- Kruszelnicki, Karl. "Hybrid Auto-Immune Women 3". ABC Science.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)
