Acetyl chloride
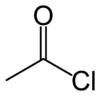
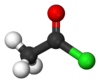
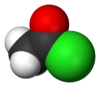
Acetyl chloride, also known as ethanoyl chloride, is an acid chloride (also known as an acyl chloride) derived from acetic acid (ethanoic acid). It has the formula CH3COCl and it belongs to the class of organic compounds called acyl halides. The chemical structure of acetyl chloride is shown at right. At room temperature and pressure, it is a clear colorless liquid. Acetyl chloride does not exist in nature, because contact with water would hydrolyze it into acetic acid and hydrogen chloride. In fact, if handled in open air it gives of white smoke owing to the hydrolysis from the moisture in the air. The smoke is actually gaseous hydrogen chloride which forms small droplets in the air with water vapour.
It is chemically synthesized by the reaction of acetic acid with thionyl chloride.
and is used as a reagent for acetylation in the synthesis or derivatization of chemical compounds. Examples of acetylation reactions include acylation processes such as esterification (see below) and the Friedel-Crafts reaction).
- CH3COCl + HO-CH2-CH3 → CH3-COO-CH2-CH3 + H-Cl
Frequently such acylations are carried out in the presence of a base such as pyridine, triethylamine, or DMAP, which as catalysts help to promote the reaction and as bases neutralize the resulting HCl.
Acetylation is the introduction of an acetyl group via acylation using a reactant such as acetyl chloride or acetic anhydride. An acetyl group is an acyl group having the formula
-C(=O)-CH3.
For further information on the types of chemical reactions compounds such as acetyl chloride can undergo, see acyl halide.