Silibinin
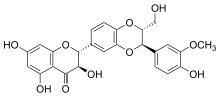 | |
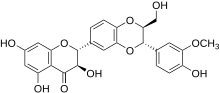 Silibinin A and silibinin B structures | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral and Intravenous |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.168 |
| Chemical and physical data | |
| Formula | C25H22O10 |
| Molar mass | 482.441 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Silibinin (INN), also known as silybin (both from Silybum, the generic name of the plant from which it is extracted), is the major active constituent of silymarin, a standardized extract of the milk thistle, containing a mixture of flavonolignans consisting of silibinin, isosilibinin, silychristin, silidianin, and others. Silibinin itself is a mixture of two diastereomers, silybin A and silybin B, in approximately equimolar ratio.[1] Silibinin is used in pure forms as a medication, and more frequently as an active ingredient in milk thistle–derived herbal supplements.
Medical uses
[edit]For approved drug preparations and parenteral applications in the treatment of Amanita mushroom poisoning, the water-soluble silibinin-C-2',3-dihydrogensuccinate disodium salt is used. In 2011, the same compound also received Orphan Medicinal Product Designation for the prevention of recurrent hepatitis C in liver transplant recipients by the European Commission.[2]
Silibinin is available in many EU countries for treatment of toxic liver damage (e.g., as an intravenous formulation used in death cap poisoning) or as adjunctive therapy in chronic hepatitis and cirrhosis.[citation needed][3][4]
There is limited evidence to support use of silibinin-containing products as a supplement in people with chronic liver disease. A systematic review and meta-analysis concluded that silymarin does not affect all-cause mortality in persons with cirrhosis, but it may help prevent liver-related mortality in those patients.[5] There is mixed evidence for silibinin as an anti-inflammatory agent in alcohol-related liver disease or non-alcoholic fatty liver disease, and trials continue.[6] There is little evidence to support a meaningful antiviral effect of milk thistle in chronic hepatitis C.[7][8]
Potential medical uses
[edit]Silibinin is under investigation to see whether it may have a role in cancer treatment (e.g., due to its inhibition of STAT3 signalling).[9]
Silibinin has a number of potential mechanisms that could benefit the skin. These include chemoprotective effects from environmental toxins, anti-inflammatory effects, protection from UV-induced photocarcinogenesis, protection from sunburn, protection from UVB-induced epidermal hyperplasia, and DNA repair for UV-induced DNA damage (double strand breaks).[10] Studies on mice demonstrate a significant protection on chronic unpredictable mild stress (CUMS)–induced depressive-like behavior on mice[11] and increased cognition in aged rats as a result of consuming silymarin.[12]
Due to its immunomodulatory,[13] iron-chelating, and antioxidant properties, this herb has the potential to be used in beta-thalassemia patients who receive regular blood transfusions and suffer from iron overload.[14]
Pharmacology
[edit]Poor water solubility and bioavailability of silymarin led to the development of enhanced formulations. Silipide (trade name Siliphos, not to be confused with the water treatment compound of the same name, a glass-like polyphosphate containing sodium, calcium magnesium and silicate, formulated for the treatment of water problems), a complex of silymarin and phosphatidylcholine (a phospholipid in lecithin), is about 10 times more bioavailable than silymarin.[15] An earlier study had concluded Siliphos to have 4.6 fold higher bioavailability.[16][non-primary source needed] It has been also reported that silymarin inclusion complex with β-cyclodextrin is much more soluble than silymarin itself.[17] There have also been prepared glycosides of silybin, which show better water solubility and even stronger hepatoprotective effect.[18]
Silymarin, like other flavonoids, has been shown to inhibit P-glycoprotein-mediated cellular efflux.[19] The modulation of P-glycoprotein activity may result in altered absorption and bioavailability of drugs that are P-glycoprotein substrates. It has been reported that silymarin inhibits cytochrome P450 enzymes and an interaction with drugs primarily cleared by P450s cannot be excluded.[20]
Toxicity
[edit]Silibinin and all the other compounds found in silymarin, especially silychristin, blocked the MCT8 transporter according to one in vitro study.[21] There is no published clinical information showing silymarin or silibinin cause any thyroid problems. In fact, one clinical trial found that silymarin actually helped prevent thyroid suppression that is often caused by the drug lithium.[22]
There is limited research on milk thistle and silymarin in pregnant humans. However, the one known clinical trial found only benefits, including but not limited to effectively treating intrahepatic cholestasis of pregnancy.[23][24] Silymarin is also devoid of embryotoxic potential in animal models.[25][26]
A phase I clinical trial in humans with prostate cancer designed to study the effects of high dose silibinin found 13 grams daily to be well tolerated in patients with advanced prostate cancer with asymptomatic liver toxicity (hyperbilirubinemia and elevation of alanine aminotransferase) being the most commonly seen adverse event.[27]
Biotechnology
[edit]Silymarin can be produced in callus and cell suspensions of Silybum marianum and substituted pyrazinecarboxamides can be used as abiotic elicitors of flavolignan production.[28]
Biosynthesis
[edit]The biosynthesis of silibinin A and silibinin B is composed of two major parts, taxifolin and coniferyl alcohol.[29][30] Coniferyl alcohol is synthesized in milk thistle seed coat. Starting with the transformation of phenylalanine into cinnamic acid mediated by phenylalanine ammonia-lyase.[31] Cinnamic acid will then go through two rounds of oxidation by trans-cinnamate 4-monooxygenase and 4-coumarate 3-hydroxylase to give caffeic acid. The meta position alcohol is methylated by caffeic acid 3-O-methyltransferase to produce ferulic acid. From ferulic acid, the production of coniferyl alcohol is carried out by 4-hydroxycinnamate CoA ligase, cinnamoyl CoA reductase, and cinnamyl alcohol dehydrogenase. For taxifolin, its genes for the biosynthesis can be overexpressed in flowers as the transcription is light dependent. The production of taxifolin utilizes a similar pathway as for synthesizing p-coumaric acid followed by three times of carbon chain elongation with malonyl-CoA and cyclization by chalcone synthase and chalcone isomerase to yield naringenin. Through flavanone 3-hydroxylase and flavonoid 3'-monooxygenase, taxifolin is furnished. To merge taxifolin and coniferyl alcohol, taxifolin can be translocated from the flower to the seed coat through symplast pathway. Both taxifolin and coniferyl alcohol will be oxidized by ascorbate peroxidase 1 to enable the single electron reaction to couple two fragments generating silybin (silibinin A + silibinin B).

References
[edit]- ^ Davis-Searles P, Nakanishi Y, Nam-Cheol K, et al. (2005). "Milk Thistle and Prostate Cancer: Differential Effects of Pure Flavonolignans from Silybum marianum on Antiproliferative End Points in Human Prostate Carcinoma Cells" (PDF). Cancer Research. 65 (10): 4448–57. doi:10.1158/0008-5472.CAN-04-4662. PMID 15899838.
- ^ Rottapharm|Madaus. Media Communications Legalon®. Retrieved March 6, 2017.
- ^ Federico A, Dallio M, Loguercio C (Jan 2017). "Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years". Molecules. 22 (2): 191. doi:10.3390/molecules22020191. PMC 6155865. PMID 28125040.
- ^ Yarnell E (2010). Natural Approach to Gastroenterology (2nd ed.). Wenatchee: Wild Brilliance Press. p. 1701. ISBN 978-1933350066.
- ^ Saller R, Brignoli R, Melzer J, Meier R (2008). "An updated systematic review with meta-analysis for the clinical evidence of silymarin". Forschende Komplementärmedizin. 15 (1): 9–20. doi:10.1159/000113648. PMID 18334810. S2CID 23468345.
- ^ de Avelar CR, Nunes BV, da Silva Sassaki B, Dos Santos Vasconcelos M, de Oliveira LP, Lyra AC, et al. (March 2023). "Efficacy of silymarin in patients with non-alcoholic fatty liver disease - the Siliver trial: a study protocol for a randomized controlled clinical trial". Trials. 24 (1): 177. doi:10.1186/s13063-023-07210-6. PMC 10000352. PMID 36899430. Art. No. 177.
- ^ Nelson A (31 August 2022). Khatri M (ed.). "Does Milk Thistle Help Your Liver?". WebMD. Retrieved 31 December 2023.
- ^ Yang Z, Zhuang L, Lu Y, Xu Q, Chen X (2014). "Effects and tolerance of silymarin (milk thistle) in chronic hepatitis C virus infection patients: a meta-analysis of randomized controlled trials". BioMed Research International. 2014: 941085. doi:10.1155/2014/941085. PMC 4163440. PMID 25247194. Art. ID 941085.
- ^ Bosch-Barrera J, Sais E, Cañete N, Marruecos J, Cuyàs E, Izquierdo A, et al. (May 2016). "Response of brain metastasis from lung cancer patients to an oral nutraceutical product containing silibinin". Oncotarget. 7 (22): 32006–32014. doi:10.18632/oncotarget.7900. PMC 5077992. PMID 26959886.
- ^ Singh RP, Agarwal R (September 2009). "Cosmeceuticals and silibinin". Clinics in Dermatology. 27 (5): 479–484. doi:10.1016/j.clindermatol.2009.05.012. PMC 2767273. PMID 19695480.
- ^ Thakare VN, Patil RR, Oswal RJ, Dhakane VD, Aswar MK, Patel BM (February 2018). "Therapeutic potential of silymarin in chronic unpredictable mild stress induced depressive-like behavior in mice". Journal of Psychopharmacology. 32 (2): 223–235. doi:10.1177/0269881117742666. PMID 29215318. S2CID 3292948.
- ^ Sarubbo F, Ramis MR, Kienzer C, Aparicio S, Esteban S, Miralles A, et al. (March 2018). "Chronic Silymarin, Quercetin and Naringenin Treatments Increase Monoamines Synthesis and Hippocampal Sirt1 Levels Improving Cognition in Aged Rats". Journal of Neuroimmune Pharmacology. 13 (1): 24–38. doi:10.1007/s11481-017-9759-0. PMID 28808887. S2CID 255272480.
- ^ Balouchi S, Gharagozloo M, Esmaeil N, Mirmoghtadaei M, Moayedi B (August 2014). "Serum levels of TGFβ, IL-10, IL-17, and IL-23 cytokines in β-thalassemia major patients: the impact of silymarin therapy". Immunopharmacology and Immunotoxicology. 36 (4): 271–274. doi:10.3109/08923973.2014.926916. PMID 24945737. S2CID 21176675.
- ^ Moayedi Esfahani BA, Reisi N, Mirmoghtadaei M (2015-03-04). "Evaluating the safety and efficacy of silymarin in β-thalassemia patients: a review". Hemoglobin. 39 (2): 75–80. doi:10.3109/03630269.2014.1003224. PMID 25643967. S2CID 22213963.
- ^ Kidd P, Head K (2005). "A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos)" (PDF). Alternative Medicine Review. 10 (3): 193–203. PMID 16164374. Archived from the original (PDF) on 2011-07-28. Retrieved 2010-12-14.
- ^ Barzaghi N, Crema F, Gatti G, Pifferi G, Perucca E (1990). "Pharmacokinetic studies on IdB 1016, a silybin- phosphatidylcholine complex, in healthy human subjects". Eur J Drug Metab Pharmacokinet. 15 (4): 333–8. doi:10.1007/bf03190223. PMID 2088770. S2CID 26047183.
- ^ Voinovich D, Perissutti B, Grassi M, Passerini N, Bigotto A (2009). "Solid state mechanochemical activation of Silybum marianum dry extract with betacyclodextrins: Characterization and bioavailability of the coground systems". Journal of Pharmaceutical Sciences. 98 (11): 4119–29. doi:10.1002/jps.21704. PMID 19226635.
- ^ Kosina P, Kren V, Gebhardt R, Grambal F, Ulrichová J, Walterová D (2002). "Antioxidant properties of silybin glycosides". Phytotherapy Research. 16 (Suppl 1): S33–S39. doi:10.1002/ptr.796. PMID 11933137.
- ^ Zhou S, Lim LY, Chowbay B (2004). "Herbal modulation of P-glycoprotein". Drug Metabolism Reviews. 36 (1): 57–104. doi:10.1081/DMR-120028427. PMID 15072439. S2CID 25946443.
- ^ Wu JW, Lin LC, Tsai TH (2009). "Drug-drug interactions of silymarin on the perspective of pharmacokinetics". Journal of Ethnopharmacology. 121 (2): 185–93. doi:10.1016/j.jep.2008.10.036. PMID 19041708.
- ^ Johannes J, Jayarama-Naidu R, Meyer F, Wirth EK, Schweizer U, Schomburg L, et al. (2016). "Silychristin, a Flavonolignan Derived From the Milk Thistle, Is a Potent Inhibitor of the Thyroid Hormone Transporter MCT8". Endocrinology. 157 (4): 1694–2301. doi:10.1210/en.2015-1933. PMID 26910310.
- ^ Ataei S, Mahdian MR, Ghaleiha A, Matinnia N, Nili-Ahmadabadi A (July 2023). "Silymarin Improves Thyroid Function in Lithium-treated Bipolar Patients: A Randomized, Double-blind, Placebo-controlled Pilot Study". Current Drug Therapy. 18 (3): 346–353. doi:10.2174/1574885518666230710122712. S2CID 259869834.
- ^ Soleimani V, Delghandi PS, Moallem SA, Karimi G (June 2019). "Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review". Phytotherapy Research. 33 (6): 1627–1638. doi:10.1002/ptr.6361. PMID 31069872. S2CID 148569634.
- ^ Hernández R, Nazar E (1982). "Effect of silymarin in intrahepatic cholestasis of pregnancy (preliminary communication)". Revista chilena de obstetricia y ginecología. 47 (1): 22–29. PMID 6927150.
- ^ Fraschini F, Demartini G, Esposti D (2002). "Pharmacology of Silymarin". Clinical Drug Investigation. 22 (1): 51–65. doi:10.2165/00044011-200222010-00007. S2CID 20133887. Archived from the original on 2012-10-27.
- ^ Hahn G, Lehmann HD, Kürten M, Uebel H, Vogel G (1968). "On the pharmacology and toxicology of silymarin, an antihepatotoxic active principle from Silybum marianum (L.) gaertn". Arzneimittelforschung. 18 (6): 698–704. PMID 5755807.
- ^ Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, et al. (April 2007). "A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients". Investigational New Drugs. 25 (2): 139–146. doi:10.1007/s10637-006-9019-2. PMID 17077998. S2CID 20240838.
- ^ Tůmová L, Tůma J, Megušar K, Doleža M (2010). "Substituted Pyrazinecarboxamides as Abiotic Elicitors of Flavolignan Production in Silybum marianum (L.) Gaertn Cultures in Vitro". Molecules. 15 (1): 331–340. doi:10.3390/molecules15010331. PMC 6256978. PMID 20110894.
- ^ Lv Y, Gao S, Xu S, Du G, Zhou J, Chen J (December 2017). "Spatial organization of silybin biosynthesis in milk thistle [Silybum marianum (L.) Gaertn]". The Plant Journal. 92 (6): 995–1004. doi:10.1111/tpj.13736. PMID 28990236.
- ^ Prasad RR, Paudel S, Raina K, Agarwal R (May 2020). "Silibinin and non-melanoma skin cancers". Journal of Traditional and Complementary Medicine. 10 (3): 236–244. doi:10.1016/j.jtcme.2020.02.003. PMC 7340873. PMID 32670818.
- ^ Barros J, Escamilla-Trevino L, Song L, Rao X, Serrani-Yarce JC, Palacios MD, et al. (April 2019). "4-Coumarate 3-hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase". Nature Communications. 10 (1): 1994. Bibcode:2019NatCo..10.1994B. doi:10.1038/s41467-019-10082-7. PMC 6491607. PMID 31040279.
Further reading
[edit]- "Milk thistle (Silybum marianum)". Mayo Clinic. Archived from the original on 5 January 2012.
- Morazzoni P, Bombardelli E (1994). "Silybum marianum (cardus marianus)". Fitoterapia. 66: 3–42.
- Saller R, Meier R, Brignoli R (2001). "The use of silymarin in the treatment of liver diseases". Drugs. 61 (14): 2035–63. doi:10.2165/00003495-200161140-00003. PMID 11735632. S2CID 27948885.
External links
[edit]- Silymarin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
